Evaluation of the Effect of Rosuvastatin and Fenofibrate Alone and in Combination on Chronic Unpredictable Mild Stress Induced Depression in Wistar Rats
Shivangi Sharma1, Aaliya Bari1, Rishi Pal1, Satyendra Kumar Singh2, Amod Kumar Sachan1, Rakesh Kumar Dixit1 and Rajendra Nath1*
1Department of Pharmacology & Therapeutics, King George’s Medical University, India
1Department of Centre for Advanced Research (CFAR), King George’s Medical University, India
Submission: March 08, 2021; Published: April 14, 2021
*Corresponding author: Rajendra Nath, Professor, Department of Pharmacology &Therapeutics, King George’s Medical University, Lucknow, India
How to cite this article: Shivangi S, Aaliya B, Rishi P, Satyendra K S, Amod K S. Evaluation of the Effect of Rosuvastatin and Fenofibrate Alone and in Combination on Chronic Unpredictable Mild Stress Induced Depression in Wistar Rats. Open Access J Neurol Neurosurg 2021; 15(1): 555904. DOI: 10.19080/OAJNN.2021.15.555904.
Abstract
Introduction: Depression is ranked by WHO as the single largest contributor to global disability (7.5% of all years lived with disability in 2015). Despite current anti-depressants, depression is constantly on the rise. Efficacy and safety are a concern with the long-term use of antidepressants. The need of the time is to explore further options with better patient compliance, more potent and efficacious, and with fewer side effects. Rosuvastatin and Fenofibrate have shown antidepressant effects in past studies, which requires further exploration, therefore this research is designed to evaluate the antidepressant effect of these drugs on chronic unpredictable mild stress induced depression in Wistar rats.
Methods: Experimental study was conducted using a total of 30 Wistar rats divided into 5 groups randomly. Depression was induced in all 5 groups by chronic unpredictable mild stress applied for a period of 4 weeks. Group I have given 1ml saline, Group II Rosuvastatin 20mg/kg body weight, Group III Fenofibrate 200mg/kg body weight, Group IV was given a combination of Rosuvastatin 20mg/kg and Fenofibrate 200mg/kg, Group V has given standard drug Imipramine 20mg/kg body weight. All drugs were given orally and started one week before subjecting rats to a daily stressor and continued till the stress was given for the next 4weeks. After 4weeks of stress, a Forced swimming test and Sucrose preference test were performed to assess the effect of drugs on depression.
Result: Both Rosuvastatin and Fenofibrate showed a significant antidepressant effect in behavioural tests for depression. Results were statistically significant (p<0.05).
Conclusion: Both Rosuvastatin and Fenofibrate showed promising antidepressant effects, though furthermore extensive research required to establish these findings
Keywords:Rosuvastatin; Fenofibrate; Imipramine; Chronic unpredictable mild stress; Depression; Forced swimming test; Sucrose preference test; Wistar rats
Introduction
Globally, depression is the top cause of illness and disability among young and middle-aged populations [1]. People with depression are 1.52 times more likely to die than the general population, probably due to their untreated mental or physical health problems [2], as nearly half of all cases of depression remain undetected for years, and approximately 15 percent of depressive patients particularly young and elderly men die of suicide. Poor mental health can be a precursor to or a consequence of Chronic Non-Communicable Diseases (NCDs) (cardiovascular diseases, mental health disorders, diabetes, and cancer) [3,4]. The etiology of depression is not clear and various factors have been suggested in relation to its pathophysiology like
i. genetic factors,
ii. biogenic amines-serotonin, nor-epinephrine, and dopamine,
iii. alteration in hormonal regulation,
iv. alteration in sleep,
v. alteration in neurophysiology and
vi.psychosocial factors [5].
Serotonin is a major amine involved in the pathophysiology of depression. Selective serotonin reuptake inhibitors as main antidepressant drugs further support the role of serotonin [5]. There are several disadvantages with available antidepressant- [i] Onset of action of antidepressant effect takes around 4–6 weeks [6,7], [ii] Majority of patients do not respond at all to currently available antidepressant [8], [iii] several side effects decrease the patient’s compliance to therapy [9–11]. Hence, research is required for exploring new drugs with better efficacy and safety and fewer side-effects. Rosuvastatin [12-17] and Fenofibrates [18] have shown antidepressant effects in past studies and require further exploration, therefore this research is designed to evaluate the antidepressant effect of these drugs on chronic unpredictable mild stress-induced depression in Wistar rats.
Materials and Methods
Animals
Thirty adult Wistar rats of either sex weighing 160-200 grams were procured from CSIR-IITR, Gheru Campus, Lucknow, India. The animals were housed in an Institutional animal house under standard conditions of housing- room temperature of 24-27°C and humidity of 60-65% with 12-hour light and dark cycle. The food was given in the form of dry pellets and water ad libitum. The study protocol has been approved by the Institutional Animal Ethical Committee (Reference no. 121/IAEC/2019), of King George’s Medical University Lucknow, India, till approval the rats were acclimatized to a new environment. The maintenance of the animals and the experimental procedures were in the accordance with the guiding principles of the committee for the purpose of control and supervision of experimentation on animals (CPCSEA), Govt. of India.
Drugs
Rosuvastatin tablets from Mankind pharmaceuticals, used in the dose of 20mg/kg b.w. [19]
Fenofibrate tablets from USV pvt ltd. India, used in dose of 200mg/kg b.w p.o. [20]
Imipramine tablets from Intas pharmaceuticals, in dose of 20mg/kg b.w p.o.[21]
All drugs administered orally using feeding Cannula (gavage tube) with distilled water as vehicle.
Experimental design
The Wistar rats were given two-four weeks to acclimatize to the environment of the animal house. A total of thirty Wistar rats were used in the study and they were distributed randomly into five groups with six rats (n = 6) in each group. All rats were subjected to Four weeks of unpredictable mild stressors. The vehicle and drugs were given to all respective Groups one week prior to subjecting daily stressors and continued till the stress was given i.e., for the next 4weeks, (including Group I)
Group I: (non-treated stress group) given 1ml saline orally
Group II: Rosuvastatin 20mg/kg body weight (b.w) per oral (p.o)
Group III: Fenofibrate 200mg/kg b.w p.o.
Group IV: Combination of Rosuvastatin 20mg/kg b.w and Fenofibrate 200mg/kg b.w p.o.
Group V: standard drug Imipramine 20mg/kg b.w p.o.
Parameters measured
After 4weeks of CUMS (Table 1) and drug administration is over, the rats are then subjected to Behavioral Tests for assessing drug effect on depressed state- Forced Swimming Test (FST) and Sucrose Preference Test (SPT)
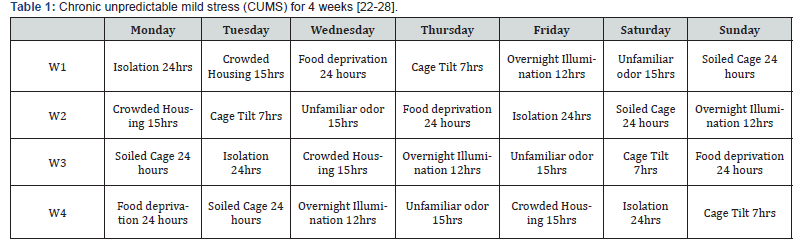
FST- The duration of immobility was recorded during the last 5 min of the 6min swimming session.
SPT- Sucrose preference ratio (SPR) is calculated for each rat.
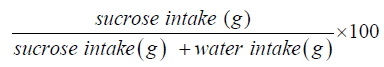
Forced swimming test (FST) [22-25]
FST is done after exposing rats of all groups to 4weeks of CUMS along with drug administration. Apparatus- Transparent cylindrical jar of dimensions (50 cm high × 20 cm diameter). It is filled with water at Room temperature. The height of water in the jar is kept such that the hind limbs of a rat can just touch the base of the jar. A pre-test session is carried out for making rats familiar with the process. It is conducted 24hr before the main test. In pre-test each rat of each group is allowed to swim for 15 mins, no measurements are taken. After the swim, each rat is dried with towels and heated for 15 min before being returned to their home cages. The next day FST is performed- Each rat is made to float in the jar for 6 minutes. The duration of immobility was recorded during the last 5 min of the 6min swimming session. Rat is considered Immobile when rat floats in an upright position, making only small movements to keep its head above water. After each swim rats dried with towels & heated for 15 min before being returned to their home cages. Principle- Initially the rat makes effort to escape and makes movements for the same. The more depressed is rat the earlier it quits making escape efforts and lies immobile. So, by calculating the duration of immobility the antidepressant effect of drugs can be compared [26,27].
Sucrose preference test (SPT) [23,24,28]
SPT is done after exposing rats of all groups to 4weeks of CUMS along with drug administration.
Sucrose solution (1% w/v) is prepared. Each rat is placed in a separate cage and marked appropriately according to the Group it belongs.Training- Rats are trained for 2days to adapt to sucrose solution (1% w/v)-
Day1- 2 bottles of sucrose solution placed in each cage for 24hrs.
Day2- 1 sucrose solution bottle, 1 water bottle placed in each cage for next 24hrs.
On SPT Day- Rats are deprived of food & water for 5hrs and after 5hrs rat in each cage is provided with 1 sucrose solution bottle, 1 water bottle for next 24hrs.
Principle- Depressed rats lose interest in sweet sucrose solution which they otherwise relished [Anhedonia]. More is depression lesser is sucrose consumption. So, by calculating SPR the antidepressant effect of drugs can be assessed.
After 24hrs consumption of sucrose & water for each rat noted Sucrose preference ratio is calculated for each rat.
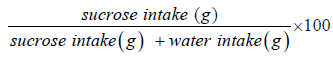
Statistical analysis
All data were expressed as Mean±SEM for six rats in each group. One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used for statistical analysis. It was carried out using the SPSS software package, version 21.0. P-value < 0.05 was considered as significant.
Results
Drugs effect on depression assessed by behavioural tests:
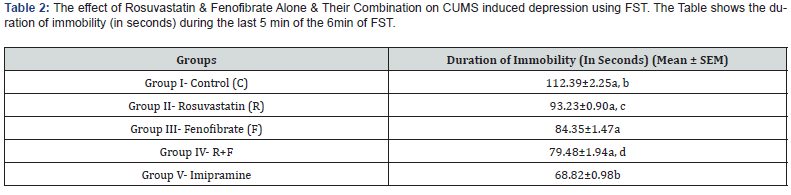
The results were depicted as Mean ± SEM in Table2 and graphically represented in Figure 1. From the Table 2 for FST, the mean and standard error of the mean (SEM) for Group I to Group V for Immobility in Rats in FST test, using One Way ANOVA the p-value is <0.05 at 95% confidence interval, showing a statistically significant relationship among variables. Applying the Post hoc Dunnett Multiple Comparison Test, Group I to Group IV were compared with Group V the p-value is <0.001 showing a statistically significant relationship.
When each group was compared with Group III, the p-value is <0.001 with the control group (Group I) and imipramine group (Group V). Comparing the Group III group with Group II the p-value is <0.05. Comparing Group III with Group IV the p-value is >0.05 (Table 2, Figure 1). Results are expressed as Mean ± SEM (n-6). One-way ANOVA followed by Dunnett’s multiple comparison test. Applying One Way ANOVA the p-value is <0.05 at 95% confidence interval (the statistically significant relationship between the variables).
a; p<0.001 compared to group V
b; p<0.001 compared to group III
c; p<0.05 compared to group III
d; p>0.05 compared to group III

Sucrose preference test (SPT)
SPT was conducted on rats from Group I to Group V and sucrose preference ratio (SPR) was calculated, and the result depicted as Mean ± SEM in Table3 and graphically represented in Figure 2. From the Table 3, the mean and standard error of the mean (SEM) for Group I to Group V, using One Way ANOVA the p-value is <0.05 at 95% confidence interval, showing a statistically significant relationship. Applying the Post hoc Dunnett Multiple Comparison Test, Group I to Group IV were compared with Group V, the p-value is <0.001 when compared with Group I and Group II, the p-value is <0.01 with Group III and compared with Group IV p-value is <0.05. When each group was compared with Group III, the p-value is <0.001 when compared with Group I, the p-value is <0.05 with Group II, Group IV, and Group V, showing a statistically significant relationship with Group III (Table 3, Figure 2).
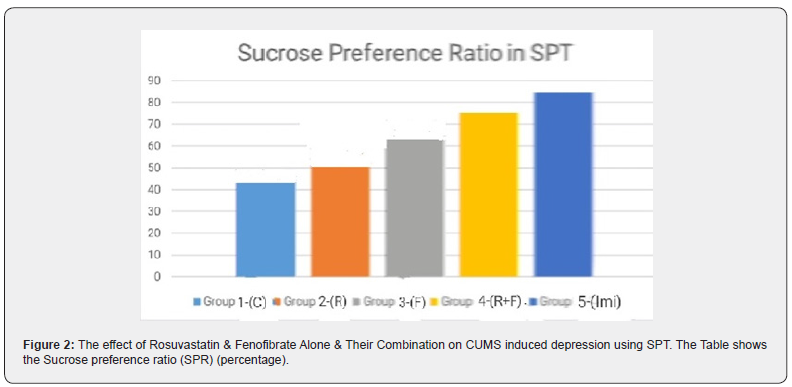
Results are expressed as Mean ± SEM (n-6). One-way ANOVA followed by Dunnett’s multiple comparison test. Applying One Way ANOVA the p-value is <0.05 at 95% confidence interval (the statistically significant relationship between the variables). a; p<0.001 compared to Group V
b; p<0.01 compared to Group V
d; p<0.05 compared to Group III
e; p<0.001 compared to Group III

Discussion
Almost every individual must have gone through some depressive phase in his/her life. Some are able to cope with it on their own, some resort to counselling and alternative treatment modalities, some end up being on long-term/lifetime of antidepressants, and some unlucky ones are not even able to address it and silently succumb to it. Under the current scenario with increased globalization, rapid socio-demographic transition, with stressful busy lives, the cases of depression are continuously increasing [29]. Despite the drugs available, globally depression is on the rise, resulting in increased disability and mortality. This indicates there is a justifiable requirement for further exploration and understanding of the neuropath physiology of depression with efforts at discovering novel effective drugs. The present study is an attempt in the same direction to further explore the pathophysiology of depression as well as to explore the pleiotropic effects of Rosuvastatin and the beneficial effect of Rosuvastatin and Fenofibrate in managing depression. This study was conducted to assess the anti-depressant effect of test drugs Rosuvastatin and Fenofibrate alone and in combination using approved behavioral models for depression assessment. In studies conducted in the past, Rosuvastatin and Fenofibrate have shown positive results when used for depression [12-18,20,30-32].
In this study 30, Adult Wistar rats were used. Rats were randomly divided into 5 groups with 6 rats in each group. Rats were allowed to acclimatize to the new environment for a period of 1 to 2 weeks.
The behavioral tests used for assessment of depression arearei. Forced Swim Test [26-28]
ii. Sucrose Preference Test [24,28]
Group I was a non-treated CUMS subjected group i.e. was given no drug but only stressors for 4weeks along with 1ml of saline daily for 5weeks (starting 1 week prior to start of CUMS and continued till 4 weeks of CUMS), Group II was given Rosuvastatin daily for 5weeks (starting 1 week prior to start of CUMS and continued till 4 weeks of CUMS) with 4week CUMS, Group III Fenofibrate daily for 5weeks (starting 1 week prior to start of CUMS and continued till 4 weeks of CUMS) with 4 weeks of stress, Group IV was combination group with both Rosuvastatin and Fenofibrate given daily for 5weeks (starting 1 week prior to start of CUMS and continued till 4 weeks of CUMS) along with CUMS, Group V was standard drug group and was given Imipramine daily for 5weeks (starting 1 week prior to start of CUMS and continued till 4 weeks of CUMS) with 4 weeks of stress and results of test drug group were compared with standard drug group.
In FST duration of immobility of rat was observed during the last 5mins of 6min FST
Duration of immobility (in seconds) (Mean ± SEM)
Control (most depressed) 112.39±2.25 > Rosuvastatin 93.23±0.90 > Fenofibrate 84.35±1.47 > Combination of R and F 79.48±1.94 > Imipramine 68.82±0.98 (Table 2, Figure 1).
A rat that is most depressed shows maximum immobility and shows the least despair to escape. From the above results, the Control Group (I) which is given no drug but only CUMS for 4weeks shows maximum immobility (depression). Imipramine is used as a standard drug. It is a tricyclic antidepressant (TCA). Imipramine acts by inhibiting the reuptake of both serotonin and norepinephrine. This is carried out by inhibiting Norepinephrine transporter (NET) and serotonin transporter (SERT) located at neuronal/platelet membrane at low and therapeutically attained concentrations. Reuptake inhibition results in increased concentration of the amines in the synaptic cleft in both CNS and periphery [33]. Imipramine Group (V) rats are hence least depressed and so show the least immobility, followed by Combination drug Group (IV) (R+F) which shows an additive effect for antidepressant activity in FST. Fenofibrate Group (III) shows a better result than Rosuvastatin Group (II), which can be attributed to the ability of Fenofibrate to cross BBB while Rosuvastatin doesn’t show such activity.
In SPT sucrose preference ratio was calculated during 24hrs of SPTSucrose
preference ratio (percentage) (Mean ± SEM)
Control 45.96±4.45 (least) (most depressed) < Rosuvastatin 51.57±6.30 < Fenofibrate 63.21±2.43 < Combination of R and F 75.28±1.88 < Imipramine 84.76±2.27 (Table 3, Figure 2). The rat that is depressed shows the least interest in drinking sucrose solution, so drinks the least amount of sucrose [Anhedonia]. From the above results Control Group (Group I) which was subjected to CUMS and was given no drug, drinks the least amount of sucrose solution, hence showing maximum depressive activity. Imipramine being an antidepressant, hence Group V shows maximum SPR and least depressive activity. Fenofibrate is PPAR (Peroxisome Proliferator Activated Receptor) α agonist. It is also a fibric acid analog. Fibrates bind to PPARα and reduce triglycerides and increase HDL-C through PPARα-mediated action [34]. Recently, more and more Fenofibrate-induced pharmacological effects on the CNS are being reported [20,31,32]. In previous studies, Fenofibrate has shown anti-depressant activity. Fenofibrate is a selective agonist of PPAR-α, it has been previously reported that WY14643, another selective agonist of PPAR-α, produced antidepressant-like effects in mice by activating the BDNF signalling pathway [18].
Rosuvastatin is an HMG-CoA reductase inhibitor. This is commonly used and is a potent statin. In a patient with severe hypercholesterolemia, it causes a greater reduction in Low- Density Lipoprotein-C partly due to its longer persistence in the plasma, and in patients with raised Triglyceride levels, it raises High-Density Lipoprotein-Cholesterol by 15–20% (greater rise than other statins) [35]. Statins in addition to their cholesterollowering action are known to possess many cholesterol independent actions including a favorable effect on vascular endothelium [36]. Moreover, there is emerging data indicating that statins exert neuroprotective and antioxidant actions [30]. Past studies investigating the effects of statins specifically on mood have reported mixed findings with some studies showing that statins in fact may protect against the risk of depression [12- 17].
Conclusion
Keeping in view the result obtained in the present study, the following conclusions may be drawn regarding the potential effectiveness of test drugs against Depression: Both Rosuvastatin & Fenofibrate have shown antidepressant effect. Fenofibrate was found to be more effective than Rosuvastatin. The combination of both test drugs showed slightly favorable results over their individual use. These results are encouraging in showing the additional anti-depressant effect of Statins & Fibrates apart from their cholesterol lowering effect, but details of the complete mechanism have yet not been explored. Therefore, further experiments are required to elucidate the exact mechanism of action. Also, more specific and longer duration animal and human studies are required to further substantiate the finding of the present study.
Funding
No funding was received for conducting this research
Acknowledgment
Authors are grateful to Dr. SC Sharma, Technical Assistant, Institutional Animal House of our University. Authors are also grateful to Mr. Ashok Kumar, senior lab technician and Mr. Shyam, lab attendant of our Department.
References
- Saxena S, Krug EG, Chestnov O (2014) World Health Organization, editors. Preventing suicide: a global imperative. Geneva: World Health Organization.
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, et al. (2014) Comprehensive Meta-Analysis of Excess Mortality in Depression in the General Community Versus, Am J Psychiatry 171(4): 453-462.
- Mathers CD, Loncar D (2006) Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLOS Med 3(11): e442.
- Patel V, Chatterji S, Chisholm D, Ebrahim S, Gopalakrishna G, et al. (2011) Chronic diseases and injuries in India. Lancet 377: 413-428.
- Sadock BJ, Sadock VA (2009) Kaplan & Sadock's synopsis of psychiatry. 10th New Delhi: Wolters Kluver. pp. 527-533.
- PL Delgado (2004) How antidepressants help depression: mechanisms of action and clinical response. Journal of Clinical Psychiatry 65(4): 25-30.
- C Taylor, AD Fricker, LA Devi, I Gomes (2005) Mechanisms of action of antidepressants: from neurotransmitter systems to signalling pathways. Cellular Signalling 17(5): 549-557.
- C Crisafulli, C Fabbri, S Porcelli (2011) Pharmacogenetics of antidepressants, Frontiers in Pharmacology (2): 1-21.
- T Sharma, LS Guski, N Freund, PC Gøtzsche (2016) Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ 352: i65.
- P S Masand, S Gupta (2002) Long-term side effects of newer generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Annals of Clinical Psychiatry 14(3): 175-182.
- I Schweitzer, K Maguire, C Ng (2009) Sexual side-effects of contemporary antidepressants: review. Australian and New Zealand Journal of Psychiatry 43(9): 795-808.
- Stafford L, Berk M (2011) The use of statins after a cardiac intervention is associated with reduced risk of subsequent depression: proof of concept for the inflammatory and oxidative hypotheses of depression? J Clin Psychiatry 72(9): 1229-1235.
- Feng L, Tan CH, Merchant RA, Ng (2008) TP: Association between depressive symptoms and use of HMG-CoA reductase inhibitors (statins), corticosteroids and histamine H (2) receptor antagonists in community-dwelling older persons: a cross-sectional analysis of a population-based cohort. Drugs Aging 25(9): 795-805.
- Yang CC, Jick SS, Jick H (2003) Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med 163(16): 1926-1932.
- Otte C, Zhao S, Whooley MA (2012) Statin use and risk of depression in patients with coronary heart disease: longitudinal data from the Heart and Soul Study. J Clin Psychiatry 73(5): 610-615.
- Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, et al. (2010) Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom 79(5): 323-325.
- Dutcher JP, Logan T, Gordon M, Sosman J, Weiss G, et al. (2000) Phase II trial of interleukin 2, interferon-alpha, and 5-fluorouracil in metastatic renal cell cancer: a cytokine working group study. Clin Cancer Res 6(9): 3442-3450.
- Jiang B, Huang C, Zhu Q, Tong LJ, Zhang W (2015) WY14643 produces anti-depressant-like effects in mice via the BDNF signaling pathway. Psychopharmacology (Berl) 232: 1629-1642.
- Rondi S, Peddolla R, Venisetty RK (2014) Neuro, cardio, and renoprotective activities of rosuvastatin in streptozotocin-induced type 2 diabetic rats undergoing treatment with metformin and glimepiride. J Adv Pharm Technol Res 5(2): 78-83.
- Ouk T, Gautier S, Pétrault M, Montaigne D, Maréchal X, et al. (2014) Effects of the PPAR-α agonist fenofibrate on acute and short-term consequences of brain ischemia. Journal of Cerebral Blood Flow & Metabolism 34(3): 542-551.
- Prathiba J, Kumar KB, Karanth KS (1999) Effects of chronic administration of imipramine on the hyperactivity of hypothalamic-pituitary-adrenal axis in neonatal clomipramine treated rats. Indian journal of pharmacology 31(3): 225-228.
- Nirmal J, Babu CS, Harisudhan T, RamanathanM (2008) Evaluation of behavioral and antioxidant activity of Cytisus scoparius Link in rats exposed to chronic unpredictable mild stress. BMC Complement Altern Med 24: 8-15.
- Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice.: A primary screening test for antidepressants. Arch. Int, Pharmacodyn. Ther. 229(2): 327-336.
- Gawali NB, Bulani VD, Gursahani MS, Deshpande PS, Kothavade PS, et al., (2017) Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviors, and cognitive impairment by modulating the nitrergic signaling pathway. Brain research 1663: 66-77.
- Jayabalan N, Chidambaram SB, Thanukrishnan H, Muthiah R (2008) Evaluation of behavioral and antioxidant activity of Cytisus scoparius Link in rats exposed to chronic unpredictable mild stress. BMC Complementary and Alternative Medicine 8:15.
- Shalam MD, Shantakumar SM, Narasu ML (2007) Pharmacological and biochemical evidence for the antidepressant effect of the herbal preparation Trans-01. Indian Journal of pharmacology 39(5): 231.
- Kompagne H, Bárdos G, Szénási G, Gacsályi I, Hársing LG, et al., (2008) Chronic mild stress generates clear depressive but ambiguous anxiety-like behavior in rats. Behavioral brain research 193(2): 311-314.
- Bhatt S, Radhakrishnan M, Jindal A, Devadoss T, Dhar AK (2014) Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (6g) on chronic unpredictable mild stress-induced changes in behavioral and brain oxidative stress parameters in mice. Indian journal of pharmacology 46(2): 191.
- (2017) Depression and other common mental disorders: global health estimates.: World Health Organization, Geneva.
- Vaughan CJ (2003) Prevention of stroke and dementia with statins: Effects beyond lipid-lowering. Am J Cardiol 91: 23B-29B.
- Barbiero JK, Santiago R, Tonin FS, Boschen S, da Silva LM, et al. (2014) PPAR-α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 53: 35-44.
- Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, et al. (2009) The PPARα agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys 75: 870-877.
- Greden JF, Gardner R, King D, Gunhaus L, Carroll BJ, et al., (1983) Dexamethasone suppression tests in antidepressant treatment of melancholia. Arch Gen Psychiatry 40: 493-500.
- Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 13th 2017. Chapter 33. P.613-614.
- Cholesterol Treatment Trialists’ (CTT) Collaboration CTT (CTT), Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. (2010) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomized trials. Lancet 376(9753): 1670-1681.
- Miida T, Takahashi A, Ikeuchi T (2007) Prevention of stroke and dementia by statin therapy: Experimental and clinical evidence of their pleiotropic effects. Pharmacol Ther 113: 378-93.






























