Primary Tumors of the Central Nervous System: Typification of Patients Attended at a Level Iii Clinic in Santiago De Cali, Colombia During the 2015 -2017 Period
Fernando González Trujillo1*, Nini Margarita Arteaga Revelo2, Daniela Giraldo Vargas3, Maria Camila Paredes Del Castillo4, Stephania Torres Salas5, Sergio Cafiero Ballesteros6, and Simón Andrés Giraldo Oliveros7
1Clinical Neurology, Observer in Neurology-Oncology, Master Neurosciences, Comprehensive Cancer Centre, Clínica de Occidente, Santiago de Cali, Colombia
2Medical Doctor, Emergency and hospital rooms, Clínica de Occidente, Santiago de Cali, Colombia
3Internist, Universidad Fundación San Martín. Clínica de Occidente. Santiago de Cali, Colombia
4Internist, Universidad Fundación San Martín. Clínica de Occidente. Santiago de Cali, Colombia
5Internist, Universidad Fundación San Martín. Clínica de Occidente. Santiago de Cali, Colombia
6Head of the Radiation Oncology Service. Comprehensive Cancer Centre, Clínica de Occidente, Santiago de Cali, Colombia
7Statistical Research and Education, Master Epidemiology, Clínica de Occidente SA Santiago de Cali, Colombia
Submission: July 8, 2020; Published: November 10, 2020
*Corresponding author: Fernando González Trujillo, Clinical Neurology, Observer in Neurology-Oncology, Master Neurosciences, Comprehensive Cancer Centre, Clínica de Occidente, Santiago de Cali, Street 18 North # 5-34, Colombia
How to cite this article: Fernando G T, Nini M A R, Daniela G V, Maria C P D C, Stephania T S, et al. Primary Tumors of the Central Nervous System: Typification of Patients Attended at a Level Iii Clinic in Santiago De Cali, Colombia During the 2015 -2017 Period. Open Access J Neurol Neurosurg 2020; 14(3): 555890.DOI: 10.19080/OAJNN.2020.14.555890.
Abstract
Introduction: Primary solid Central Nervous System (CNS) tumours are product of the accumulation of genetic alterations that allow affected cells to evade the mechanisms that regulate cell division.
Objectives: To characterize patients with primary tumours that affect the CNS attended in 2015 and 2016 in a Comprehensive Cancer Centre.
Methodology: An observational descriptive cross-sectional study with analytical intention was performed; patients confirmed by radiology and pathology criteria were included, and lesions such as inflammatory mass and secondary metastases were excluded. The collection was made by reviewing the medical history and an association analysis of the demographic characteristics, and of the characteristics of the tumor and of the treatment was carried out against the final condition of the patient at two years, using the odds ratio and its 95% confidence interval, using the software Epi Info 7.2®.
Results: Tumours were more prevalent in men and in 60-year old or more patients, and in pre-surgical images taken using the cNMR (Contrast Nuclear Magnetic Resonance) technique. The tumours were mostly located in the Supratentorial zone and according to their class they were classified into glioblastomas, astrocytomas and meningiomas.
Conclusion: The frequency of primary solid CNS tumours increased with the greater life expectancy in the population, in addition, the need to optimize the existing surgical resections with pre-surgical and on-surgery imaging is highlighted, which positively impacts the results of complementary treatment with chemotherapy and radiotherapy, improving survival rates in patients.
Keywords: CNS neoplasm; Epidemiology; Medical Oncology
Abbreviations: C720: Malignant Spinal Cord Tumor; D334: Benign Tumor Spinal Cord; D434: Uncertain Spinal Cord Tumor; C751: Malignant Neoplasm Hypophysis; D352: Benign Pituitary Tumor; D443: Uncertain Pituitary Tumor; C710: Malignant Brain Tumor; C719: Malignant Brain Tumor; D330: Benign Supratentorial Brain Tumor; D331: Benign Infratentorial Brain Tumor; D332: Benign Brain Tumor; C709: Malignant Meninges Tumor; D320: Benign Meninges Tumor; C711: Malignant Frontal lobe Tumor; C712: Malignant Temporal Lobe Tumor; C713: Malignant Parietal Lobe Tumor; C714: Malignant Occipital Lobe Tumor; C715 : Ventricular Malignant Tumor; C716: Malignant Cerebellum Tumor; CNS: Central Nervous System; cNMR: Contrast Nuclear Magnetic Resonance; WHO: World Health Organization; ICD: 10 International Classification of Diseases 10; Ccat: Contrast Computed Axial Tomography; IMRT: Iensity Modulated Radiation Therapy; WBRT: Whole Brain Radiation Therapy; Abs: Absolute; Freq: Frequency; Rel Freq: Relative Frequency; 3D Third Dimension; DANE: National Administrative Department of Statistics
Introduction
During evolution, primary solid CNS tumours accumulate genetic alterations that allow affected cells to evade the mechanisms that regulate cell division and escape the control of the immune system [1]. The etiology is multiple and involves genetic and environmental factors that participate differently depending on the tumor subtype [1,2]. There are different hypotheses that review the risk factors and their relationships to explain the origin of these tumours, considering intrinsic factors such as social, demographic, anthropometric, hormonal, immunological, and genetic variables; and external factors such as ionizing radiation, electromagnetic fields, diet, infections, pesticides, drugs; only the causal relationship with the factor of exposure to ionizing radiation has been established [2]. Recording information about tumours that affect the CNS is not easy, the data is not organized. Histologically they are highly variable and have marked differences that are difficult to analyse in epidemiological studies. Epidemiological databases use the classification of the World Health Organization (WHO), which mainly classifies them according to histological variables. When the goal of collecting information about these tumours does not fit a precise variable, the job is difficult. The variables that are applied for the sample collection range from age, gender, geographic location, race, histology to variables of survival and response to treatments, a very heterogeneous content. The annual incidence figures for primary solid CNS tumours range from 8.5 to 21.4 cases per 100,000 inhabitants [2, 3]. In 2012, the WHO reported that there were 139,608 men and 116,605 women affected worldwide [3]. In the United States of America, the Cancer Society reported in 2015 22,850 cases and 15,320 deaths related to brain tumours [3]. In children, they are the second most frequent cause of cancer, comprising between 15% and 25% of paediatric tumours cases [3]. Depending on the histological type, different types of primary solid CNS tumours predominate at certain ages. In paediatrics, ependymomas, medulloblastomas, and primitive neuroectodermal tumours are frequent, and in adults younger than 40 years old, benign glial tumours have a higher incidence, while in adults older than 40 years old, genomic changes appear evolving towards malignant glial type tumor varieties such as the glioblastoma [1,3].
There is a direct correlation between age and location of the tumor, and for example, tumours of the posterior fossa predominate in the preadolescent population, and supratentorial tumours increase towards adulthood [3]. Furthermore, men are more likely to develop these tumours, and for example, data from the United States population report a male/female ratio of 1.5/1 [3]. Neuroepithelial tumours predominate in men and meningiomas predominate in women [1,2], while the incidence of pituitary tumours is similar in both genders [1]. There are variations by race and by geographic place of residence that were largely defined by the quality of care and by the opportunity of the population to access to health systems. For example, the Japanese have been reported to have low incidences of malignant brain tumours compared to citizens of Northern Europe. On the other hand, in the United States, the white population had more gliomas than the Afro-descendant population, and the trend was similar with meningiomas [1,3]. In Latin America, gliomas predominated, accounting for 55.4% of brain tumours [4].
Statistical records for the Latin American population showed that the incidence of tumours is higher in men, and thus, for example, it was reported that in Cuba it is 5.1 in men and 3.6 in women, in Brazil it was 6.4 in men and 4.8 in women , and in Uruguay 6.2 in men and 4.0 in women [4]. The survival variable is correlated to the age and to the histological grade of the tumor; the elderly have lower survival rates. There are exceptions in the paediatric population in children under 3 years old, who have low survival compared to infants over 3 years old and up to 14 years old. There are general data that have reported a 5-year survival of 20% in the United States, grouping all ages and tumor histological types [1,3,5]. Compared with glioblastoma multiforme, malignant brain tumours and other benign or mixed histological types have better survival rates, but when glioblastoma was assessed between different age groups, no difference was found [1,6].
In Latin America, the reported mortality rates were higher in countries such as Argentina, Brazil and Chile, without statistical significance [4]. The objective of this study was to characterize the patients attended between 2015 and 2017 in a Comprehensive Cancer Centre in southwestern Colombia, in the city of Cali, specifically patients with primary tumours that affect the CNS, in order to provide decision-makers and the care staff of the Comprehensive Cancer Centre evidence on the behavior of these pathologies and its comparison with information at local, national and international levels.
Methodology
An observational descriptive cross-sectional study with analytical intention was carried out at a Comprehensive Cancer Centre in southwestern Colombia. This study sought to characterize the occurrence of primary solid CNS tumours according to the demographic characteristics of the patients, class, histological location of the tumor and final condition of the patient. The study subjects were patients with primary solid CNS tumours attended between September 1, 2015 and September 30, 2017 at a Comprehensive Cancer Centre located in Santiago de Cali. The following codes of the International Classification of Diseases 10 (ICD 10) were defined: C720, D334, D434, C751, D352, D443, C710, C719, D330, D331, D332, C709, D320, C711, C712, C713, C714, C715, C716. Patients with primary solid tumor in the CNS confirmed by radiology and pathology criteria were included in the study, resulting in a total of 285 patients attended in the study period. Exclusion criteria included lesions that behave like an inflammatory mass, metastases from primary tumours located outside the nervous system, or masses that infiltrated the nervous system by contiguity from nearby tumours, excluding a total of 195 patients. Furthermore, patients with loss of information in their clinical records of more than 10% were excluded, a criterion by which a patient was excluded for whom it was not possible to find pathology data and/or only suspected diagnostic impression was obtained by radiology. In the end, a census sample of 89 patients remained.
The information was collected through a clinic history review, digitizing the information in a pre-validated spreadsheet according to the operationalization of the variables, which was designed by the research group and completed by internal doctors who rotated through the oncology service and who were duly trained in the institution’s information system and the proper completion of the database. To guarantee the quality of the records, 10% of the records in the database were randomly audited, contrasting them with the clinic histories. This audit was performed by a Radiation Oncologist and a Medical Specialist in Clinical Neurology and trained in Neuro-oncology. To answer the objective of the present investigation, an analysis of absolute and relative frequencies of the treated patients was performed, according to demographic and tumor-related characteristics such as: sex and age group, type of presurgical images, tumor location and tumor class, and also according to characteristics of the treatment and the final condition such as: chemotherapy, type of chemotherapy, radiotherapy and type of radiotherapy, surgery, degree of resection of the tumor and final condition of the patient. This analysis was presented using tables. Subsequently, the association of the demographic characteristics of the patient, the tumor and the treatment was evaluated against the final condition of the patient after two years (alive or deceased), by means of contingency tables, taking as the mean of association the odds ratio and determining its significance through its 95% confidence interval. Statistical and epidemiological processing was performed using the Epi Info 7.2® software.
Results
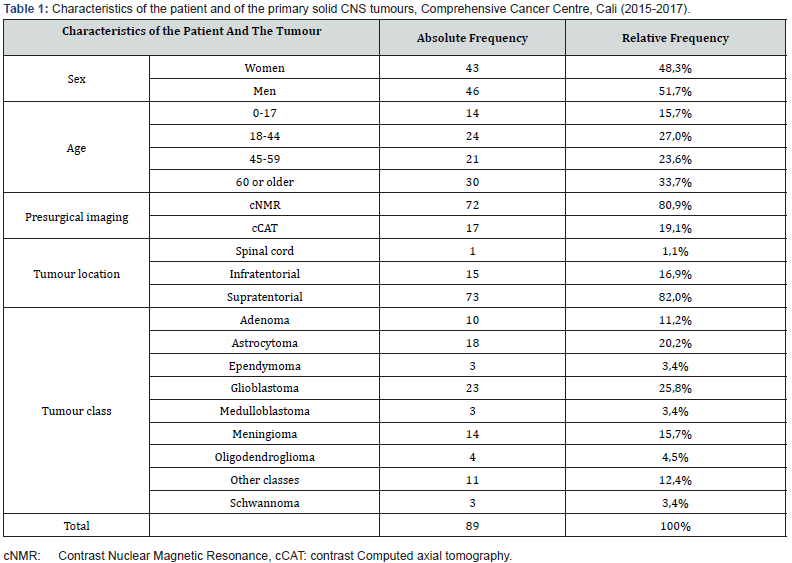
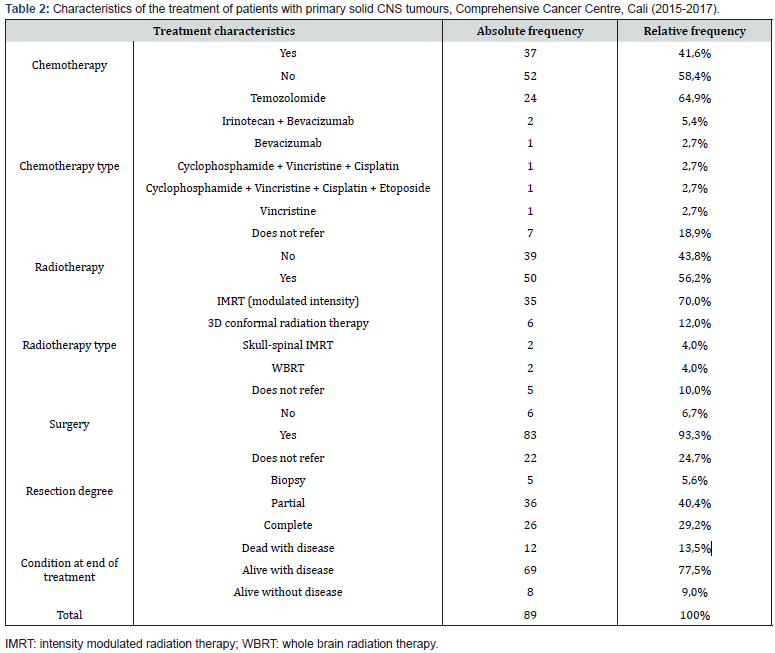
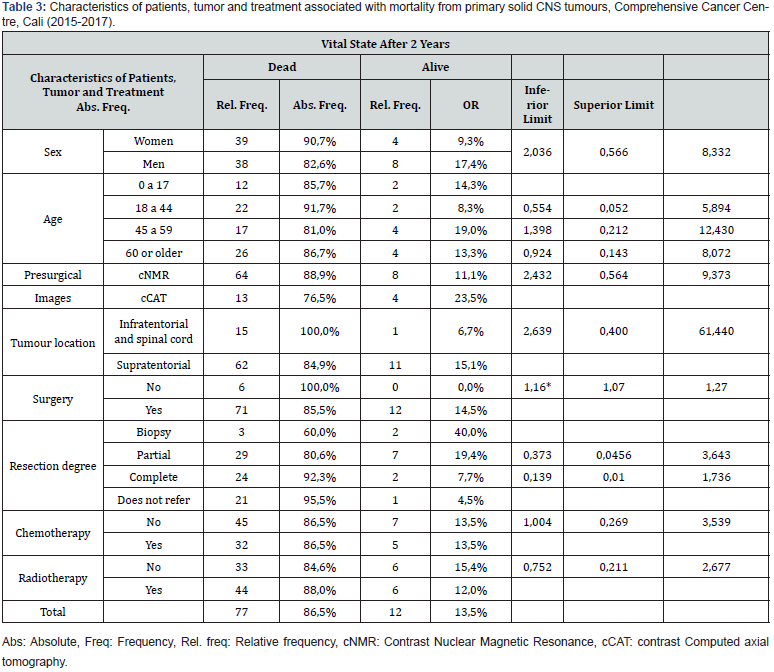
A higher prevalence of primary solid CNS tumours was found in men, people over 60 years of age, and in pre-surgical images taken by means of the cNMR (Contrast nuclear magnetic resonance) technique. Moreover, most tumours were located in the supratentorial zone, and according to their class, glioblastomas, astrocytomas and meningiomas were the most common, totaling 61.8% of the total (Table 1). Four out of ten patients received chemotherapy as part of their treatment. The most frequent type of chemotherapy was Temozolomide. Furthermore, more than half of the patients received radiotherapy as part of their treatment, and the most frequent types of radiotherapy were IMRT (intensity-modulated radiotherapy), followed by the 3D conformal radiation therapy. Nine out of ten patients were treated surgically, and when evaluating the degree of tumor resection, the majority reported partial or complete resection (69.7%). Furthermore, the percentage of mortality at two years from diagnosis was 13.5% and of cancer cure was 9.0% (Table 2). Mortality was more prevalent in women, in young people between 18 and 44 years old, in those with preoperative cNMR images, in patients with tumours of infratentorial or spinal cord location, in those who did not undergo surgical procedures, and in those who underwent complete resection and radiotherapy. Mortality was found to be the same between patients who received and those who did not receive chemotherapy. On the other hand, among the factors evaluated, only surgery was significant, indicating that those who did not undergo surgery had a 16% higher risk of dying two years later compared to patients who underwent this procedure (Table 3).
Discussion
Regarding sex, there was a higher incidence of primary solid CNS tumours in men. This is how reports from the population in the United States reported the male-female ratio at 1.5 / 1 [3]. Regarding the figures in Central and South America, the report by Dr. Piñeros et al. showed similar results, finding a higher incidence in men [4]. The global data for world population presented by the Canadian group of Dr. Jette et al did not report a statistically significant difference in the total estimate by gender for brain tumours in relation to gender: 15.80 / 100,000 person-years ( 95% CI: 10.30–24.24) in women and 14.33 / 100,000 person-years (95% CI: 10.07–20.38) in men [7]. The prevalence and incidence of primary solid CNS tumours increased with the increase in life expectancy in the population, a fact demonstrated by a study that showed the high prevalence and low mortality that was found between 1990 and 2015 [8]. Currently, these types of tumours are defined as a public health problem, being the most common cause of death from cancer in children and the second cause in young adults [9]. The population in our study were people older than 60 years old, and it should be clarified that the institution where the study was carried out had better consolidated the adult oncology service during the study period, a fact that could bias the data obtained.
The pre-surgical images were taken in most of the patients by means of NMR, thanks to which better-defined images of the lesions were obtained. The interpretation of the results was made by radiologist doctors trained in resonance reading. Diagnostic imaging resonance is considered the preferred standard method for diagnosing and monitoring brain tumours, which increases diagnostic reliability compared to studies such as computed tomography. Currently, there are defined diagnostic criteria based on special sequences of magnetic resonance to assess the response and progression of treated tumours [10]. The primary solid CNS tumours were located mainly in the supratentorial zone, and meningiomas were the most common benign tumor. Moreover, according to their histological class, glioblastomas, astrocytomas and meningiomas were the most frequent. Piñeros et al. reported that the most frequent histological type of tumor in Latin America were the gliomas with figures of up to 55.4% [4]. In the reviewed literature, malignant gliomas were found to be the most frequent, representing 80% of primary brain tumours [11], a figure like that found in our study. On the other hand, meningiomas are the most frequent benign tumours in the nervous system, representing between 13 and 26% [11], being the third most frequent type of tumor within our study sample.
Four out of ten patients received chemotherapy as part of their treatment; the most frequent type of chemotherapy was Temozolomide. 50.5% of the tumours in the study population sample were glial tumours treated with temozolomide, which was the agent of choice in chemotherapy protocols. This drug was administered for both low-grade II and III tumours and highgrade glioblastoma tumours, according to the WHO classification [12,13]. More than half of the patients received radiotherapy as part of their treatment, the most frequent type of radiotherapy was the IMRT followed by the 3D conformal radiation therapy. The patients in our study received the radiotherapy treatment plan adjusted to the protocols. IMRT allows precise doses of radiation to be delivered to the tumor reducing radiation to normal perilesional tissue. This technique is preferred to treat tumours of the nervous system located close to critical structures such as the optic nerve, optic pathway, chiasm, brain stem and spinal cord [14]. Radiotherapy is a fundamental pillar in the treatment of tumours, current treatment protocols in gliomas contemplate dual treatment schemes with concomitant chemotherapy that have improved the variables of progression-free survival [12,13]. This protocol was applied in the patients of our study who presented glioblastomas.
Nine out of ten patients were treated surgically, and when evaluating the degree of tumor resection, the majority reported partial or complete resection (69.7%). The extent of surgical resection is limited by the location of the tumor in eloquent areas of the brain, by the infiltrating type of the tumor, and by the lack of margins to achieve complete resection. The degree of resection is important to improve survival variables and seizure control [15,16]. Statistical data reported 10-year survival of 91% of patients with low-grade gliomas when resection was greater than 90%, and in patients with high-grade gliomas improved survival and quality of life were achieved with extensive resections [15]. Cancer cure rate was 9.0%, which reflected very low responses to the applied treatments. The global analysis changes when each tumor is reviewed separately, for example, causing the cure variable to be affected by the biological behavior and histology of the tumor, and by age, race, socioeconomic status, and responses to the treatment regimens applied. In the United States, 5-year survival rates of 96.1% were reported for benign tumours and 34.2% for malignant tumours, while in Europe 85% survival was reported for benign tumours and 19.9% for malignant tumours [2]. Meningiomas are benign tumours with reports of long survival, and when there is complete resection there is also complete cure. The same is true for other histological types such as pituitary adenomas, schwannomas, pilocytic astrocytoma, among others [2,9].
The proportion of mortality two years after diagnosis was 13.5%, being higher in women, in young people between 18 and 44 years old, in patients with preoperative images such as NMR, in those with tumours of infratentorial location or in the spinal cord, in those who did not undergo surgical procedures, in those who had incomplete resection of the tumor, and in those who underwent radiotherapy. Only the surgery variable was statistically significant, indicating that those who did not undergo surgery had a 16% higher risk of dying two years later compared to patients who underwent surgery. The young population with brain tumours has high morbidity and mortality in relation to the adult population. Statistical data from studies in England reported that girls and young women with primary CNS tumours had higher rates in the average mortality-year compared to subjects of the same age with tumours in other body locations (in 0-14 years old age group it was 33%, in 15-24 years old age group it was 20% and in 25-49 years old group 15-24 years old it was 6%). These data are very similar to those found in men [17]. Mortality in the sample assessed at the Comprehensive Cancer Centre in Cali is low compared to the reported data. Other reports support that survival generally decreases with age at diagnosis: children and young adults generally have better survival rates for most histological types of brain tumours. Furthermore, survival in women was slightly better [2]. 84% of the patients in our study were over 18 years old, and it was found that according to the histological type, gliomas were the most frequent within this population, with glioblastoma multiforme being the most frequent. Glioblastoma is a very aggressive tumor that presents high mortality rates; for this, it was expected that in the sample assessed in our study there would be a higher mortality rate as found in global reports. Statistical reports in the United States showed that glioblastoma multiforme was the second most frequent type of tumor in the population over 55 years old, whereas pituitary tumours and meningiomas were the most frequent in the population over 20 years old [17]. These results were different from those found in our study.
The location of the tumor in the infratentorial cranial fossa and the highest mortality values in the sample corresponded to few cases; many studies have already established the correlation between age and tumor location. Tumours of the posterior fossa predominate in the preadolescent population, and the incidence of supratentorial tumours increase towards adulthood [3]. The posterior cranial fossa contains vital structures that are compacted in a reduced space, so the presence of a tumor represents great surgical difficulty and some histological types are infiltrative, making it impossible to perform surgery or obtain a biopsy, with the risk of generating great morbidity in the patient. In this type of scenarios, the results of the histological samples are of poor prognosis [18]. Mortality was the same between patients who received and those who did not receive chemotherapy. The same results were observed with radiotherapy, and only patients undergoing surgery presented better results. Previously, it was reported how surgery positively impacted survival, and how the combination of surgery and radiotherapy improved it. Furthermore, the addition of chemotherapy to treatment is beneficial for certain histological types, without significantly impacting survival when compared to other treatment regimens [19]. Additionally, we present the reports from Colombia and the southwestern region registered by the National Cancer Institute in Colombia, which published in 2017 (latest report found in the DANE databases, official government body) the cancer mortality atlas. The period between 2007 and 2013 was analyzed the total number of deaths in the country within the group of women, regardless of anatomical location, was 119,055 cases, and for the southwest, there were 21,537 cases, which represented 18% of the samples. Valle del Cauca was the third department with the most cases. There were 115,708 cases of men who died of this pathology in Colombia, and in the southwestern region there were 20,455 cases, representing 17.7%. As in women, Valle del Cauca is the third department with the highest number of reported cases. Mortality was reviewed according to the compromised system: for brain and other cancers of the central nervous system, there were 3,123 women dead in Colombia and in the southwest of the country there were 497 cases, representing 15.9% of the total. On the other hand, there were 3,650 men killed by the pathology in question in Colombia, and in the southwest, there were 2,547 cases, which is 14.9%. For both genders, Valle del Cauca is the third department in the country with the highest presentation of cases due to brain tumours [20]. In our study, higher mortality was found in women, which contrasts with the global values for the country and the south-western region, where there were no differences according to sex. Finally, the limitations of the study are specified: the reviewed medical records had incomplete reported information, and moreover, the changing contracts with the health insurers did not allow the patients to carry out the complete treatment in the comprehensive cancer centre. On the other hand, several radiology reports did not contain the tumor size volumes in the reported descriptions, and the pathology reports did not include the molecular profile results, which did not allow us to carry out this work with the latest WHO 2016 classification for brain tumours that classifies them according to the expressed molecular profile.
Conclusion
The results show that the frequency of primary solid CNS tumours increased with the longer life expectancy of the population, being considered a public health problem today. The data of this work should serve medical groups to better understand the population served and their predominant pathologies and will allow to adjust measures in treatment protocols aimed at improving the health conditions of patients. On the other hand, the need to optimize surgical resections is highlighted, with existing pre-surgical and surgical imaging aids, technologies that facilitate broad resections with less morbidity. Radiology reports should be standardized with the current criteria of the radiology committees in the area of neuro-oncology, and the pathological reports should contain the results of the tumor molecular profile [21,22]. This will allow future work to be carried out and will allow complete information on the population served. Our work provides information to the Colombian health system that allows to know the trend of presentation of brain tumours in the southwestern region of the country. We hope that this study contributes to improve patient care policies, serving as a comparative basis for work in neuro-oncology.
Acknowledgment
We would like to thank Dr. Jorge Karim Assis, Director of the Department of Research and Education of the Clínica de Occidente S.A., for allowing us to access the institutional registry system of medical records that helped to carry out this work. In addition, we would also like to thank the professional in Statistics Iliana Hernández for the support in the analysis of the registered information.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Wrensch M, Chew T, Berger MS, MinnY, Bond ML (2005) Textbook of Neuro-Oncology.Eds Mitchel S, Berger and Michael D Prados. Chapter 1: Epidemiology of brain tumors. isbn: 0-7216-8148-4 copyright © by Elsevier Inc.
- Pouchieu C, Baldi I, Gruber A, Berteaud E, Carles C, et al. (2016) Descriptive epidemiology and risk factors of primary central nervous system tumors: Current knowledge. Revue Neurologique 172(1): 46-55.
- Bruce M Lo (2015) Brain Neoplasms Updated, Medscape. Chief Editor: Brenner BE.
- Piñeros M, Sierra MS, Izarzugaza MI, Forman D (2016) Descriptive epidemiology of brain and central nervous system cancers in Central and South America. Cancer Epidemiol 44(1):S141-S149.
- Darlix A, Zouaoui S, Rigau V, Bessaoud F, Figarella-Branger D, et al. (2017) Epidemiology for primary brain tumors: a nationwide population-based study. J Neurooncol 131(3):525-546.
- Jiang H, Cui Y, Wang J, Lin S (2016) Impact of epidemiological characteristics of supratentorial gliomas in adults brought about by the 2016 world health organization classification of tumors of the central nervous system. Oncotarget 8(12):20354-20361.
- Paula de Robles, Kirsten M Fiest, Alexandra D Frolkis, Tamara Pringsheim, Callie Atta, et al. (2014) The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro-Oncology 17(6): 776-783.
- GBD 2015 Neurological Disorders Collaborator Group(2017) Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015.Lancet Neurol 16(11):877-897.
- Garcia CR, Slone SA, Dolecek TA, Bin Huang, Janna H Neltner, et al. (2019) Primary central nervous system tumor treatment and survival in the United States, 2004-2015. J Neuroonco 144(1):179-191.
- Nowosielski M, Wen PY (2018) Imaging Criteria in Neuro-oncology. Semin Neurol 38(1):24-31.
- Strong MJ, Garces J, Vera JC, Mathkour M, Emerson N, et al. (2015) Brain Tumors: Epidemiology and Current Trends in Treatment. Brain Tumors Neurooncol 1: 102.
- Miller JJ, Wick W (2018) What’s New in Grade II and Grade III Gliomas? Semin Neurol 38(1):41-49.
- Reitman ZJ, Winkler F, Elia AEH (2018) New Directions in the Treatment of Glioblastoma. Semin Neurol 38(1):50-61.
- Ospina R, Vallejo MT, Feliciano-Alfonso JE, Gómez GA, González G, et al. (2019)Updating the indications for the use of Intensity-Modulated Radiation Therapy in the Instituto Nacional de Cancerología - Colombia. Clinical protocol reported in the evidence. Rev Colomb Cancerol 23(2):7-12.
- Hervey-Jumper SL, Berger MS (2016) Maximizing safe resection of low- and high-grade glioma. J Neurooncol 130(2):269-282.
- Rennert RC, Santiago-Dieppa DR, Figueroa J, Sanai N, Carter BS (2016) Future directions of operative neuro-oncology. J Neurooncol 130(2):377-382.
- McNeill KA (2016) Epidemiology of Brain Tumors. Neurol Clin 34(4):981-998.
- Wolff JE, Rytting ME, Vats TS, Zage PE, Ater JL, et al. (2012) Treatment of recurrent diffuse intrinsic pontine glioma: the MD Anderson Cancer Center experience. J Neurooncol 106(2):391-397.
- Iwamoto FM, Reiner AS, Nayak L, Panageas KS, Elkin EB, et al. (2009) Prognosis and Patterns of Care in Elderly Patients With Glioma. Cancer 115(23):5534-5540.
- Pardo C, de Vries E, Buitrago L, Gamboa O (2017) Atlas de mortalidad por cáncer en Colombia. Cuarta edició Bogotá D. C. Instituto Nacional de Cancerología, 1.p.124.
- Nowosielski M, Wen PY(2018) Imaging Criteria in Neuro-oncology. Semin Neurol 38(1): 24-31.
- Van den Bent MJ, Weller M, Wen PY, Kros JM, Aldape K, et al. (2017) A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro Oncology 19(5):614-624.






























