First Time in Human Study: Neuroseal Dural Sealant Prevents Cerebrospinal Fluid Leakage Following Elective Cranial Surgery
Pal Barzo, Sandor Szabo2, Peter Orbay3 and Virginia Jamieson4*
1Department of Neurosurgery, University of Szeged, Albert Szent-Györgi Medical Center, Hungary
2 Department of Neurosurgery, Medical School University Debrecen, Hungary
3 National Institute of Neurosurgery, Hungary
4Kuros Biosurgery AG, Switzerland
Submission: January 24, 2020; Published: February 06, 2020
*Corresponding author: Virginia Jamieson, Kuros Biosurgery AG, Wagistrasse 25, 8952 Schlieren, SwitzerlandBrasilia, DF, Brazil
How to cite this article: Pal B, Sandor S, Peter O, Virginia J. First Time in Human Study: Neuroseal Dural Sealant Prevents Cerebrospinal Fluid Leakage Following Elective Cranial Surgery. Open Access J Neurol Neurosurg. 2020; 12(5): 555850.DOI: 10.19080/OAJNN.2020.12.555850.
Abstract
Cerebrospinal Fluid (CSF) leakage remains a significant cause of morbidity and mortality and can occur when no watertight Dural closure is achieved after neurosurgery. We carried out a retrospective clinical study to investigate the safety and effectiveness of a novel dural sealant, Neuroseal, to stop or prevent CSF leakage following elective cranial surgery. In a prospective, open, multi-center, single-arm study in 45 adult patients (n=38 completed) eligible patients received Neuroseal, a novel, colored synthetic dural sealant to augment dural closure following suturing of the dura. The performance of Neuroseal was considered effective if there was no intra operative CSF leakage evident visually or during a Valsalva maneuver up to 20 cm H2O held for 5-10 seconds from the dural repair after a maximum of two applications. The patients were followed up for any CSF leakage and secondary complication for 90 days following surgery. For the 40 patients in whom the device was properly applied, 100% intra operative sealing of the dura was achieved. No leakage was seen up to 90 days after surgery. All AEs that occurred during the study reflected the typical post-operative complications for the study population. Neuroseal was considered easy and quick to assemble as 98 % of preparations took less than 5 minutes. Ease of application was considered very good by 71 % and good by 29 % of the investigators. Neuroseal offers an easy-to-use and visible topical therapy which is safe and effective to provide a watertight dural closure as an adjunct to suturing [1,2].
Keywords: Cranial surgery; CSF leak; Dural sealant; Neuroseal; Cerebrospinal fluid
Abbreviations: AE: Adverse Event; CI: Confidence Interval; CSF: Cerebrospinal Fluid; MRI: Magnetic Resonance Imaging; PEG: Polyethylene Glycol; SAE: Severe Adverse Event; TEAE: Treatment-Emergent Adverse Event
Introduction
Cerebrospinal Fluid (CSF) leakage remains a significant cause of morbidity and mortality and occurs when no watertight dural closure is achieved after neurosurgery [3-6]. The occurrence of CSF leakage after surgery can produce serious complications such as infections, severe headaches, meningitis and neurological complications. Furthermore, the financial consequences of CSF leakage may be significant: in a study of 412 procedures in one neurosurgical department CSF leakage accounted for 22% of the total cost of the neurosurgical procedures [3]. Numerous materials and methods have been evaluated in the past decades to identify an optimal sealant to achieve watertight dural closure with as yet no consensus on a standardization of dural closure methods and assessment of outcomes. Despite advances in neurosurgical techniques to repair dural defects, the incidence of post-operative CSF leaks remains high with rates of 12.8% and 34% for infratentorial and skull-based surgeries, respectively [3].
Neuroseal, previously known as I 020805 is a novel, synthetic colored polyethylene Glycol (PEG)-based hydrogel that is an easy to use sealant that provides watertight closure as an adjunct to dural suturing following intracranial neurosurgical procedures. Neuroseal has undergone extensive biocompatibility, and pre-clinical safety and effectiveness testing. A watertight dural closure, is achieved by forming a hydrogel layer in situ that adheres to the dura, thus preventing CSF leakage and associated complications. In vitro studies showed that Neuroseal did not contribute to bacterial growth, as tested with bacillus atrophaeus, staphylococcus epidermidis, pseudomonas aeruginosa or micrococcus luteus bacteria. Two synthetic PEG based hydrogels are marketed as dural sealants (DuraSeal™ by Integra, Adherus™ by Hyperbranch). While DuraSeal™ is an effective dural sealant for supratentorial procedures [2,5] its swelling properties (130 240% volume increase in vitro) may have limited its use elsewhere. Preclinical data show Neuroseal has ideal characteristics to support its investigation as a safe and effective adjunct to sutured dural repair to provide a watertight dural closure in patients undergoing elective cranial surgery [7-8].
Materials and Methods
Patients
Pre-operative eligibility: Patients were aged 18 years or more and scheduled for an elective cranial procedure (involving surgical wound classification Class I/Clean) entailing a dural incision of at least 2cm in length or more for supratentorial location or posterior fossa approach whatever is the origin e.g. tumor, vascular indication. Non pregnant females of childbearing potential were eligible if they used acceptable birth control or abstinence until 90 days after surgery. Patients were not eligible if they required a procedure involving trans labyrinthine, transoral or any procedure that penetrated the air sinus or mastoid air cells, had symptomatic hydrocephalus, pre-existing external ventricular drainage or lumbar CSF drain, or had received radiotherapy in the planned surgical region ending within 3 months before surgery, or had systemic infection or evidence of surgical site infection, or a history of clinically significant coagulopathy including hemophilia, or were using an oral anticoagulant. Intra-operative eligibility Same as pre-operative but with a dural margin of at least 3 mm from the edges of the bony defect; on satisfactory completion of the sutured dural repair leakage of CSF was assessed visually or via Valsalva maneuver with a maximum of 20 cm H2O for 5 10 seconds. Patients were not eligible if they were unable to tolerate a Valsalva maneuver, or had an intra operative CSF shunt, or needed to use synthetic or non-autologous duraplasty material, or there was a gap of more than 2 mm after primary closure of the dura.
Study design
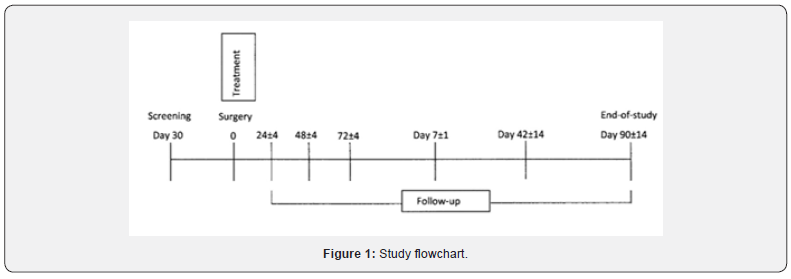
This was a prospective, open label, single arm study (Figure 1) with patients enrolled at one center in Germany (Universitätsmedizin Neurochirurgische Kinik, Berlin; and three centers in Hungary (Szegedi Tudományegyetem, Szent-Györgyi Albert Klinikai Központ, Szeged; Országos Idegtudományi Intézet, Budapest; Debreceni Egyetem Orvos és Egészségtudományi Centrum, Debrecen) from 14 February 2011 to 02 November 2011. Study conduct complied with ethical standards and the Declaration of Helsinki. Written informed consent was obtained from each participant before undergoing any study assessments.
After dural suturing, eligible patients received Neuroseal and, if necessary, autologous grafts to augment dural closure. Neuroseal was delivered from a double syringe applicator to create a fine layer over the dura, which quickly set to form a watertight seal. Patients were followed-up for any subsequent CSF leakage and secondary complications at several time points (Figure 1) or at the point of any withdrawal, whichever occurred first.
Preparation and Application of Neuroseal
Neuroseal (Figure 2) comprises three hydrogel components: a PEG-based polymer in solution containing a colorant which changes color during setting for better product visualization; a lyophilized PEG polymer; and an activator solution. Before application, the polymer solution is transferred into the lyophilized PEG polymer vial under vacuum, which ensures effective mixing. The resulting solution is transferred into one chamber of a double syringe spray applicator, and the activator solution is aspirated into the second chamber. After mixing of the 2 polymers Neuroseal needs to be applied within 45 minutes. Prior to application the spray nozzle is attached to the applicator. During application, the contents of the two chambers are mixed within the spray nozzle. A cross linking reaction between the two polymers produces a hydrolytically degradable conformal coating which has good physical adherence to the dura mater and resistance to CSF pressure. In this study, up to two applications from one kit were considered appropriate to provide an effective seal. After the dura was closed and Neuroseal had been applied, a Valsalva maneuver was performed to demonstrate a watertight seal had been achieved.

Assessments
Device performance evaluation
Patients were monitored daily for laboratory and vital signs and incidence of CSF leakage for up to 8 days following surgery or before discharge from hospital, whichever occurred first. The incidence of CSF leakage, CSF leakage with breaking skin (pseudo meningocele-related surgical intervention), and CSF leakage confirmed by clinical evaluation was monitored for 90 days (±14 days) following surgery. Moreover, diagnostic testing with MRI was performed on days 7 (±1 days) and 90 (±14 days). The performance of Neuroseal was considered effective if there was no intra operative CSF leakage during a Valsalva maneuver (up to 20 cm H2O held for 5 10 seconds) from the dural repair after a maximum of two applications. Neuroseal could be applied a second time if leakage was observed as a result of the Valsalva maneuver following the first application. Following the second application a further Valsalva maneuver was to be performed. The device failed if CSF leakage was seen intra operatively during the Valsalva maneuver (up to 20 cm H2O for 5-10 seconds) after up to two applications of Neuroseal.
Safety evaluation
Patients were monitored for Adverse Events (AEs) and Serious Adverse Events (SAEs) from the time of informed consent until the end of the study. Vital signs, physical examinations including neurological status and laboratory assessment and wound healing impairment, were performed in accordance with the study schedule of assessments. In particular patients were monitored for signs of post-operative surgical infection and unexpected neurological signs for 90 days (±14 days) following surgery. During the hospital period patients were monitored daily for up to 7 days (±1 day) or up to discharge from hospital whichever occurred first and specific follow up visits were performed on day 42 (±14 days) and at the end of the study day 90 days (±14 days) following surgery.
Device evaluation
The device was evaluated with respect to: preparation time (i.e., mixing of polymers, and transfer into double syringe); ease of application (i.e., including setting time, material run-down, spray properties, clogging, and spray nozzle exchange); gel properties (i.e., color, texture, adhesiveness, and layer thickness) and any possible failures in relation to these; and suitability of instructions for use and packaging concept, including unsterile/ sterile package.
Statistical methods
All statistical analyses were performed using SAS v9.2 or higher. The Safety Analysis Set was used for all efficacy analyses. The primary endpoint was the frequency and incidence (%) of performance failure (95% Confidence Interval [CI]). The Clopper Pearson Exact method was used to assess the incidence, and the secondary performance endpoints relating to CSF leakage. A sample size of 40 was planned on the assumption that the true incidence of intra operative CSF leakage (assuming no occurrence of intra operative CSF leakage) would be no more than 8.8%3.
Results
Patients characteristics
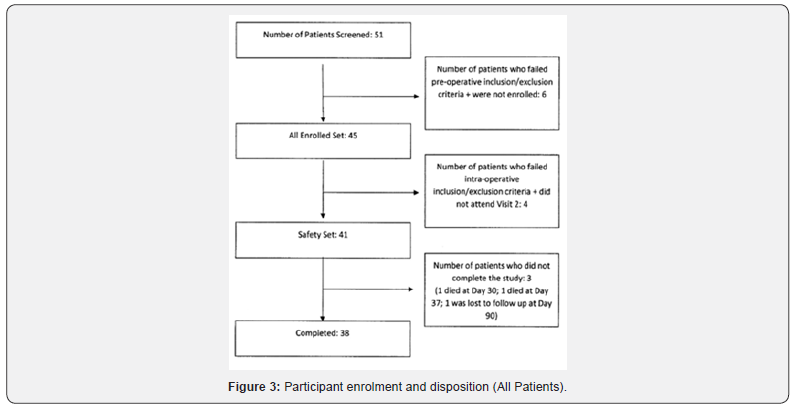
A total of 51 patient were screened, and 45 were enrolled. All 45 patients were white Caucasian and comprised 28 males and 17 females. The mean age was 48.5 years (Table 1). Patients undergoing surgery suffered a variety of lesions some which were benign such as anerysms and ateriovenous malformations and others malignant such as gliomas or astrocytomas. The majority of lesions were supratentorial lesions with 20% infratentorial or skull based. Forty-one patients received Neuroseal. Thirtynine patients received a single application from a single kit, one partient received two applications from a single kit and one patient received two applications from two separate kits, as the first application of the device was removed because it had been applied too thickly. Thirty-eight patients (84%) completed the study (Figure 3).
Device performance evaluation
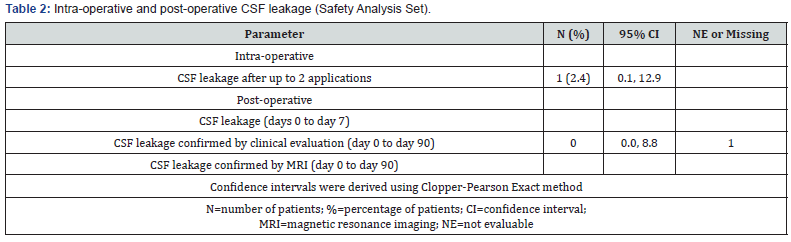
In 40 out of the 41 patients the device was properly applied, and this achieved 100% sealing of the dura intra operatively, and no leakage was seen up to 90 days after surgery (Table 2). In 1 patient, out of the 41 treated, spontaneous CSF leakage occurred following the incorrect application of the device. The material was not applied correctly to the dural suture line, leaving part of the suture line uncovered. Neuroseal was applied for a second time but spontaneous leakage continued because the investigator was unable to cover the remaining dural suture line with the residual 1 mL (4 mL out of the 5 mL of product had been used during the first application). This was considered a use error and not a device failure, as confirmed by the investigator.
Safety
Neuroseal was considered a safe adjunct to sutured dural repair during cranial surgery because all the AEs that occurred during the study reflected the typical post-operative complications for this population.
Adverse events
Symptoms of malaise (nausea, vomiting, headache and fever) were the most common post-surgery AEs, but none were considered to be related to the use of Neuroseal. Overall, 228 treatment-emergent adverse events (TEAEs), i.e., events that started or increased in severity on or after the date of surgery, were reported by 40 (97.6%) patients, of which, most were mild (92.7%), non-serious, were considered unrelated to the study device and did not lead to subject withdrawal. Two patients experienced adverse events that were considered possibly related to the device 1 case of mild wound secretion that was treated with routine wound care and 1 case of wound infection which was considered serious as it extended the hospital stay for the patient. Both resolved completely. The most common TEAEs, i.e., those that were reported by 5.0% or more of patients are summarized in Table 3.
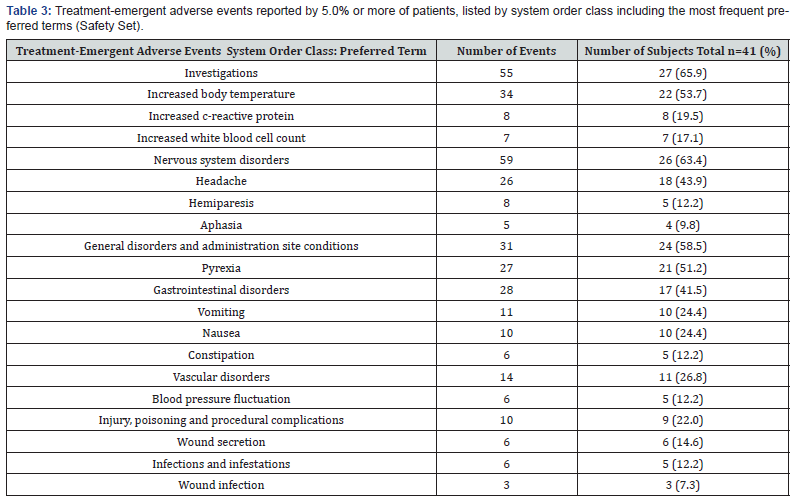
Serious adverse events
Thirteen SAEs were reported for 12 patients, of which one event (SAE 1: wound infection) was considered possibly related to the device. The other unrelated SAEs comprised aseptic meningitis, left sided hemiparesis [1], disorientation, seizure, post procedural hemorrhage, superficial wound infection (2 – one associated with hemorrhage at site of surgery), cachexia progression of disease, wound seroma. No deep wound infections were reported.
Changes in laboratory parameters
The most common clinically significant laboratory abnormalities were reductions in hemoglobin and hematocrit and increases in C reactive protein after surgery. All were considered to be an expected consequence of surgery and unrelated to the study device.
Neurological symptoms
A total of 9 (22%) patients experienced unexpected neurological symptoms/signs such as hemiparesis (two patients), seizures (two patients), photophobia (one participant), aphasia (one participant) aseptic meningitis (one participant) disorientation, impaired short term memory loss (one participant), confusion, motor dysphasia and temporal lobe epilepsy (one participant) during the study period. None of these were considered attributable to the use of Neuroseal, and most of them of them recovered during study participation.
Device evaluation
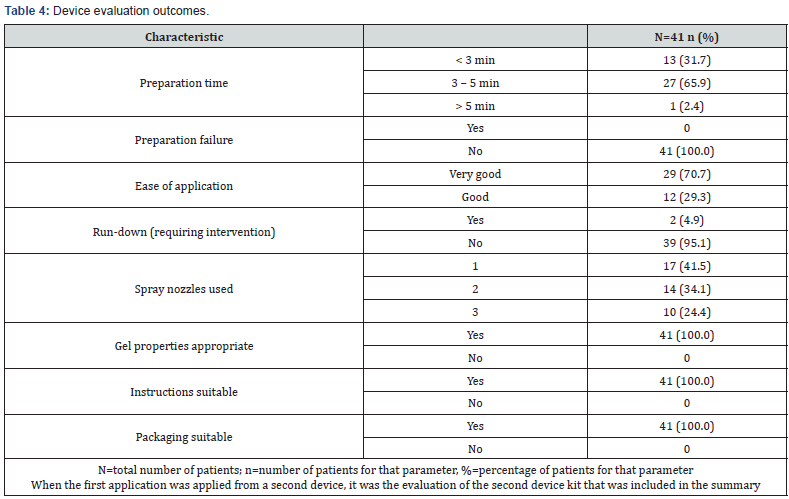
Device evaluation outcomes are summarized in Table 4. The recorded preparation time was under 5 minutes in 97% of cases and there were no preparation failures. Ease of application was classified as very good for 71% of the preparations and good for 29% of the preparation. The gel color, texture, adhesiveness and layer thickness were considered appropriate by all users. Application of the device as a fine spray produced a thin layer on sutures 30 100 mm long. The volume of material supplied in one kit (5 mL) was sufficient to apply an effective layer of Neuroseal to the suture line as shown by a median volume used of 2.1 mL All investigators considered the instructions for use and packaging concept to be suitable.
Discussion
The study population was a typical representation of patients requiring elective craniotomy procedures following a protocol that was typical for device evaluation at this stage of development. An open label design was conducted to assess the performance as the intraoperative endpoint which had a binary outcome, seal or no seal under standard test conditions of Valsalva maneuver up to CSF pressures of 20cms H2O). As with other marketed predicate devices such as Duraseal [2,5] and Adherus [1], Neuroseal demonstrated 100% sealing intra-operatively when applied over the entire suture line with up to 2 spray applications. Intra-operative sealing is well recognized as an important step in preventing post-operative CSF leaks. In this study a follow up period of 90 days was a secondary endpoint and considered an appropriate timeframe in order to detect post-operative CSF leakage and any adverse events that might be attributable to the device. In this small sample of patients there were no post-operative CSF leaks in the 40 patients receiving the device correctly applied. Literature review of the use of predicate devices, showed a post-operative leakage rate of 7.6% on Duraseal [8] and 8% (2/25) on Adherus [7], although the exact nature and site of the surgery for these trials makes any meaningful comparison difficult.
Our study did not include a composite endpoint in the protocol. In the next planned study a composite endpoint comprising intraoperative, sealing, post-operative leakage within 90 days and lack of unplanned treatments attributed to the use of the device will be included. As with any implantable device there is a risk of infection. In this study one case of superficial wound infection was reported as possibly related to the device. There were no deep wound infections. Literature comparison with other predicate devices is difficult given the different methods of assessing attribution of an adverse event (e.g. investigator alone or adjudication committee). True comparisons will require prospective randomized controlled studies.
Conclusion
This study indicates that Neuroseal offers a safe and effective, easy to use and easily visible topical therapy for obtaining a watertight dural closure following cranial surgery that extends into the post-operative period.
Acknowledgement
We acknowledge the help from the members of clinical site team and other external supporting groups that contributed to the success of this clinical study. We also acknowledge the editorial and medical writing support of Kathryn White (Cathean Ltd. Medical Writing Consultancy).
Conflict of Interest
Virginia Jamieson was an employee of Kuros Biosurgery AG and is currently a consultant to the company. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants prior to inclusion in the study.
References
- Adherus™ Autospray Dural Sealant. Summary of Safety and Effectiveness Data PMA: P130014.
- DuraSeal™ Dural Sealant System. Instructions for use.
- Grotenhuis JA (2005) Costs of post-operative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases. Surg Neurol 64: 490-493.
- Kumar A, Maartens NF, Kaye AH (2003) Evaluation of the use of Bio Glue in neurosurgical procedures. J Clin Neurosci 10(6): 661-664.
- Osburn JW, Ellenboggen RG, Chesnut RM, Chin LS, Connolly PJ, et al. (2005) A multicenter, single-blind, prospective randomized trial to evaluate the safety of a polyethylene glycol hydrogel (Duraseal Dural Sealant System) as a dural sealant in cranial surgery. World Neurosurg 78(5): 498-504.
- Schiariti M, Acerbi F, Broggi M, Tringali G, Raggi A, et al. (2014) Two alternative dural sealing techniques in posterior fossa surgery: (Polyactide-co glycolide) self-adhesive resorbable membrane versus polyethylene glycol hydrogel. Surg Neurol Int 5: 171.
- Vizirgianakis G (2009) First human clinical experience with adherus dural sealant for watertight dural closure. White Paper CS-03397-03397R2.
- Weinstein JS, Liu KC, Delashaw JB Jr, Burchiel KJ, van Loveren HR, et al. (2010) The safety and effectiveness of a dural sealant system for use with nonautologous duraplasty material. J Neurosurg 112(2): 428-433.






























