Glia Maturation Factor in the Pathogenesis of Alzheimer’s Disease
Swathi Beladakere Ramaswamy1, Sachin M Bhagavan1, Harleen Kaur1, Gema E Giler1, Duraisamy Kempuraj1,2*, Ramasamy Thangavel1,2, Mohammad Ejaz Ahmed1,2, Govindhasamy Pushpavathi Selvakumar1,2, Sudhanshu P Raikwar1,2, Smita Zaheer1, Shankar S Iyer1,2,, Raghav Govindarajan1 and Asgar Zaheer1,2
1Department of Neurology, and the Center for Translational Neuroscience, School of Medicine, University of Missouri, Columbia, MO, USA
2Harry S Truman Memorial Veterans Hospital, US Department of Veterans Affairs, Columbia, MO, USA
Submission: November 13, 2019; Published: December 17, 2019
*Corresponding author: Duraisamy Kempuraj, Department of Neurology, and the Center for Translational Neuroscience, School of Medicine, University of Missouri, Columbia, MO, USA
How to cite this article: Swathi Ramaswamy, Sachin Bhagavan, Harleen Kaur, Gema E Giler, Duraisamy Kempuraj etc.,all. Glia Maturation Factor in the Pathogenesis of Alzheimer’s Disease. Open Access J Neurol Neurosurg. 2019; 12(3): 555840. DOI: 10.19080/OAJNN.2019.12.555840.
Abstract
Alzheimer’s Disease (AD) is a neurodegenerative and neuroinflammatory disease characterized by the presence of extracellular Amyloid Plaques (APs) and intracellular Neurofibrillary Tangles (NFTs) in the brain. There is no disease modifying therapeutic options currently available for this disease. Hippocampus, entorhinal cortex (Broadmann area 28), perirhinal cortex (Broadmann area 35) and insular cortices are areas within the brain that are first ones to be severely affected in AD. Neuroinflammation is an important factor that induces neurodegeneration in AD. Glia Maturation Factor (GMF), a proinflammatory factor plays a crucial role in AD through activation of microglia and astrocytes to release proinflammatory mediators in the brain. Through immunohistochemical studies, we have previously shown that GMF is highly expressed in the vicinity of APs and NFTs in AD brains. Glial Fibrillary Acidic Protein (GFAP), reactive astrocytes, ionized calcium binding adaptor molecule-1 (Iba-1) labelled activated microglia and GMF immunoreactive glial cells are increased in the entorhinal cortical layers especially at the sites of APs and Tau containing NFTs indicating a role for GMF. Overexpression of GMF in glial cells leads to neuroinflammation and neurodegeneration. Inhibition of GMF expression reduces neurodegeneration. Therefore, we suggest that GMF is a novel therapeutic target not only for AD but also for various other neurodegenerative diseases.
Keywords: Alzheimer’s disease Amyloid plaques Neurofibrillary tangles Glia maturation factor Hippocampal formation Neurodegenerative diseases Glial activation Neuroinflammation Chemokines
Abbrevations: AD: Alzheimer’s Disease; Aps: Amyloid Plaques; GMF: Glia Maturation Factor; GFAP: Glial Fibrillary Acidic Protein; NFTs: Neurofibrillary Tangles; CNS: Central Nervous System; MS: Multiple Sclerosis; EAE: Experimental Autoimmune Encephalomyelitis; MAPKs: Mitogen Activated Protein Kinases; ROS: Reactive Oxygen Species; iNOS: Nitric Oxide Synthase; LAMP1: Lysosome Associated Membrane Protein1; TNF: Tumor Necrosis Factor-alpha
Introduction
Alzheimer’s Disease (AD) is a chronic progressive neurological disorder affecting 5.8 million Americans and 35 million individuals worldwide (Alzheimer’s disease Association, Chicago, IL). Extracellular Amyloid Plaques (APs) and intracellular Neurofibrillary Tangles (NFTs) are the hallmarks of AD. NFTs are made up of abnormally phosphorylated Tau proteins and its number is closely associated with clinical symptoms in AD patients [1-10]. Entorhinal cortex is involved in memory formation, retrieval, and extinction. Hippocampus is important for learning and memory functions. Together with hippocampal formation, the entorhinal cortex forms the major part of medial temporal lobe that is involved in AD and has dense NFTs formation and amyloid deposits. Entorhinal cortex and hippocampus are severely affected, atrophied and inflamed in AD patients. Memory loss is one of the earliest symptoms in AD patients due to the destruction of entorhinal cortex projections and the perforant pathways to the hippocampal formation [2,3]. In this mini review, we discuss on the role of Glia Maturation Factor (GMF) in the pathogenesis of AD.
Glia maturation factor and Alzheimer’s disease
Recent studies suggest that inflammation plays a critical role in the onset and progression of neuroinflammatory and neurodegenerative diseases including AD [11-15]. GMF, a brain protein was previously isolated and characterized in our laboratory is mainly found to be localized in glial cells and in some neurons in the Central Nervous System (CNS) [16-18]. GMF in excessleads to the death of neurons by inducing neuroinflammation in neurodegenerative diseases including AD, Parkinson’s Disease (PD), Multiple Sclerosis (MS) and its animal model Experimental Autoimmune Encephalomyelitis (EAE) (Figure 1) [19-28].
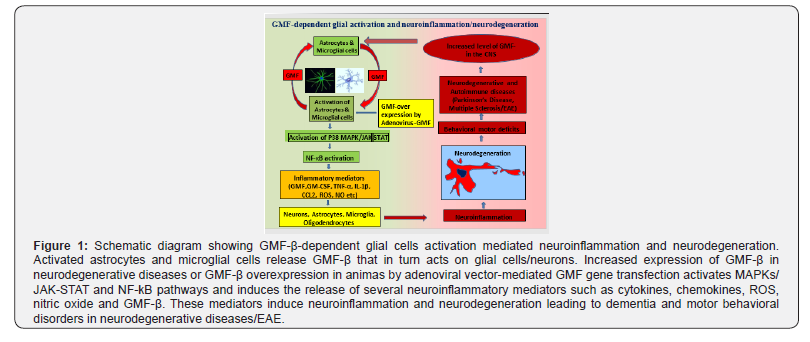
There is specific upregulation of GMF expression in glial cells associated with APs and NFTs in the entorhinal cortical layers and hippocampus of AD brains [2,3]. We believe that GMF is a novel therapeutic target and therefore inhibition of GMF expression can inhibit the onset and progression of neurodegenerative diseases including AD [29-33]. GMF and Glial Fibrillary Acidic Protein (GFAP) are increasingly expressed in the hippocampus and entorhinal cortex of AD brain especially at the sites of APs and NFTs [2-6]. During normal aging process hippocampus and entorhinal cortex are subjected to widespread oxidative stress, decreased antioxidant function and enhanced expression of GFAP. GMF accelerates and potentiates these processes and makes the neuronal cells more susceptible to degeneration in neuroinflammatory conditions [3-7,17,34,35]. Neuronal death in AD is mediated by APs and NFTs. Other factors such as local inflammatory infiltrates including glial cells (microglia/ astrocytes) and release of inflammatory molecules contribute to neuroinflammation and disease severity [4,16,36-38]. AD pathogenesis involves sustained neuroinflammation by glial activation that produces proinflammatory cytokines, chemokines, free radicals such as reactive oxygen species/ reactive nitrogen species, and activation of transcription factor nuclear factorkappa B (NF-κB) and Mitogen Activated Protein Kinases (MAPKs) [4,7,16,36-38]. Mitochondrial dysfunction plays a crucial role in the development and progression of AD. Mitochondria are the main sites for Reactive Oxygen Species (ROS) production. Increased expression of Inducible Nitric Oxide Synthase (iNOS) around the plaques has been shown to contribute to the oxidative stress in AD brains. Uncoupling Proteins (UCPs) are inner mitochondrial proteins that protect neurons by reducing the production of free radicals [7]. UCP2 and UCP4 are down regulated in AD brains with upregulation of GMF expression in the glial cellsalong with increased iNOS and NF-κB activities thereby indicating that GMF plays a proinflammatory role in the pathogenesis of AD probably by promoting mitochondrial dysfunction through down regulation of the UCPs. Mitochondrial dynamics in AD may be regulated on one hand by UCPs through their action on FASN/fatty acid synthase and on other hand by GMF through its action on the NLRP3 inflammasome [7].
Recently, we have shown that in human AD brains, GMF colocalizes with the NLRP3 inflammasome and the autophagosome markers Lysosome Associated Membrane Protein1 (LAMP1) and autophagic protein sequestosome1 (SQSTM1)/p62, clearly pointing to the role of GMF in increased Aβ level in AD aggregates. Analysis of human AD brain tissue sections from the temporal cortex showed besides GMF increased expression of the inflammasome components NACHT, LRR and PYD domains-containing protein 3 (NLRP3) and caspase-1 along with the products interleukin- 1beta (IL-1β and IL-18 [39]. The co-localization of inflammasome components and pro-inflammatory cytokines with GMF was found in the vicinity and periphery of APs and NFTs. GMF and apolipoprotein E4 (ApoE4) are strongly expressed and coassociated in the APs and reactive astrocytes surrounding APs in AD brains [40]. These results show that GMF and ApoE4 should together be contributing to the neuropathological changes associated with AD. GMF enhances astrocyte activation through secretion of Granulocyte-Macrophage-Colony Stimulating Factor (GM-CSF) [19]. High level expression of GMF in activated glial cells further augments chemoattraction, proliferation, activation and release of inflammatory mediators IL1, IL-33, Tumor Necrosis Factor-alpha (TNF-a), Macrophage Inflammatory Proteins-1 beta (MIP-1β), complement protein C1q, class II Major Histocompatibility Complex (MHC) proteins, 12-lipoxygenaseand chemokine CX3C and exacerbating the pathogenesis of AD. This reflects the paracrine and/or autocrine signaling by GMF [1-4,16,41,42]. Increased expression of GMF in association with β-amyloid (Aβ) has been shown to amplify the deleterious inflammation propagated by the NLRP3 inflammasome causing mitochondrial dysfunction, and further that GMF and A β synergize in bringing upon this dysfunction by changing mitochondrial dynamics through alterations in fission and fusion proteins [43].
Conclusion
Increased expression of GMF by glial cells in the temporal cortex of AD brain suggests GMF’s proinflammatory role of neurodegeneration in the pathogenesis of AD. Furthermore, we suggest that GMF is a novel therapeutic target not only for AD but also for various other neurodegenerative diseases.
Acknowledgement
This work was supported by the National Institutes of Health (NIH) Grant AG048205 and Veterans Affairs Research Career Scientist Award to Asgar Zaheer
References
- Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A (2012) Expression of glia maturation factor in neuropathological lesions of Alzheimer's disease. Neuropathol Appl Neurobiol 38(6): 572-581.
- Stolmeier D, Thangavel R, Anantharam P, Khan MM, Kempuraj D, et al. (2013) Glia maturation factor expression in hippocampus of human Alzheimer's disease. Neurochem Res 38: 1580-1589.
- Thangavel R, Kempuraj D, Stolmeier D, Anantharam P, Khan M, et al. (2013) Glia maturation factor expression in entorhinal cortex of Alzheimer's disease brain. Neurochem Res 38(9): 1777-1784.
- Thangavel R, Van Hoesen GW, Zaheer A (2009) The abnormally phosphorylated tau lesion of early Alzheimer's disease. Neurochem Res 34(1): 118-123.
- Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, et al. (2014) Alzheimer's Disease: Evidence for the Expression of Interleukin-33 and Its Receptor ST2 in the Brain. J Alzheimers Dis 40(2): 297-308.
- Zaheer S, Thangavel R, Sahu SK, Zaheer A (2011) Augmented expression of glia maturation factor in Alzheimer's disease. Neuroscience 194: 227-233.
- Thangavel R, Kempuraj D, Zaheer S, Raikwar S, Ahmed ME, et al. (2017) Glia Maturation Factor and Mitochondrial Uncoupling Proteins 2 and 4 Expression in the Temporal Cortex of Alzheimer's Disease Brain. Front Aging Neurosci 9: 150.
- Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A (2009) Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in Alzheimer's disease. Neuroscience 160(2): 427-433.
- Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A (2008) Modular and laminar pathology of Brodmann's area 37 in Alzheimer's disease. Neuroscience 152(1): 50-55.
- Thangavel R, Van Hoesen GW, Zaheer A (2008) Posterior parahippocampal gyrus pathology in Alzheimer's Neuroscience 154(4): 667-676.
- Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, et al. (2016) Neuroinflammation Induces Neurodegeneration. J Neurol Neurosurg Spine 1(1): 1003.
- Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Dhaliwal AS, et al. (2019) Brain Injury-Mediated Neuroinflammatory Response and Alzheimer's Disease. Neuroscientist 16: 1073858419848293.
- Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, et al. (2019) Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer's Front Cell Neurosci 13: 54.
- Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, et al. (2017) Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer's Disease Pathogenesis. Front Neurosci 11: 703.
- Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, et al. (2017) Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front Cell Neurosci 11: 216.
- Lim R, Miller JF, Zaheer A (1989) Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci U S A 86: 3901-3905.
- Khan MM, Zaheer S, Thangavel R, Patel M, Kempuraj D, et al. (2015) Absence of Glia Maturation Factor Protects Dopaminergic Neurons and Improves Motor Behavior in Mouse Model of Parkinsonism. Neurochem Res 40(5): 980-990.
- Lim R, Zaheer A, Lane WS (1990) Complete amino acid sequence of bovine glia maturation factor beta. Proc Natl Acad Sci U S A 87(14): 5233-5237.
- Zaheer A, Yorek MA, Lim R (2001) Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res 26(12): 1293-1299.
- Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, et al. (2007) A novel role of glia maturation factor: induction of granulocyte-macrophage colonystimulating factor and pro-inflammatory cytokines. J Neurochem 101(2): 364-376.
- Zaheer A, Knight S, Zaheer A, Ahrens M, Sahu SK, et al. (2008) Glia maturation factor overexpression in neuroblastoma cells activates glycogen synthase kinase-3beta and caspase-3. Brain Res 1190: 206-214.
- Zaheer S, Wu Y, Yang X, Ahrens M, Sahu SK, et al. (2012) Clinical course of myelin oligodendrocyte glycoprotein 35-55 induced experimental autoimmune encephalomyelitis is aggravated by glia maturation factor. Neurochem Int 60(3): 215-219.
- Zaheer S, Wu Y, Bassett J, Yang B, Zaheer A (2007) Glia maturation factor regulation of STAT expression: a novel mechanism in experimental autoimmune encephalomyelitis. Neurochem Res 32(12): 2123-2131.
- Khan MM, Kempuraj D, Thangavel R, Zaheer A (2013) Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem Int 62 (4): 379-388.
- Kempuraj D, Thangavel R, Selvakumar GP, Ahmed ME, Zaheer S, et al. (2018) Mast Cell Proteases Activate Astrocytes and Glia-Neurons and Release Interleukin-33 by Activating p38 and ERK1/2 MAPKs and NF-kB. Mol Neurobiol 56(3): 1681-1693.
- Selvakumar GP, Iyer SS, Kempuraj D, Ahmed ME, Thangavel R, et al. (2018) Molecular Association of Glia Maturation Factor with the Autophagic Machinery in Rat Dopaminergic Neurons: A Role for Endoplasmic Reticulum Stress and MAPK Activation. Mol Neurobiol 56(6): 3865-3381.
- Selvakumar GP, Iyer SS, Kempuraj D, Raju M, Thangavel R, et al. (2018) Glia Maturation Factor Dependent Inhibition of Mitochondrial PGC-1alpha Triggers Oxidative Stress-Mediated Apoptosis in N27 Rat Dopaminergic Neuronal Cells. Mol Neurobiol 55(9): 7132-7152.
- Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, et al. (2015) Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins alphaSynuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators. PLoS One 10(8):
- Raikwar SP, Thangavel R, Dubova I, Selvakumar GP, Ahmed ME, et al. (2018) Targeted Gene Editing of Glia Maturation Factor in Microglia: a Novel Alzheimer's Disease Therapeutic Target. Mol Neurobiol 56(1): 378-393.
- Raikwar SP, Kikkeri NS, Sakuru R, Saeed D, Zahoor H, et al. (2019) Next Generation Precision Medicine: CRISPR-mediated Genome Editing for the Treatment of Neurodegenerative Disorders. J Neuroimmune Pharmacol 14(4): 608-641.
- Raikwar SP, Thangavel R, Dubova I, Ahmed ME, Selvakumar PG, et al. (2018) Neuro-Immuno-Gene- and Genome-Editing-Therapy for Alzheimer's Disease: Are We There Yet? J Alzheimers Dis 65(2): 321-344.
- Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Kempuraj D, et al. (2019) CRISPR/Cas9 Editing of Glia Maturation Factor Regulates Mitochondrial Dynamics by Attenuation of the NRF2/HO-1 Dependent Ferritin Activation in Glial Cells. J Neuroimmune Pharmacol 14(4): 537-550.
- Fan J, Fong T, Chen X, Chen C, Luo P, et al. (2018) Glia maturation factor-beta: a potential therapeutic target in neurodegeneration and neuroinflammation. Neuropsychiatr Dis Treat 14: 495-504.
- Zaheer S, Wu Y, Yang X, Thangavel R, Sahu SK, et al. (2012) Efficient downregulation of glia maturation factor expression in mouse brain and spinal cord. Neurochem Res 37(7): 1578-1583.
- Zaheer S, Thangavel R, Wu Y, Khan MM, Kempuraj D, (2013) Enhanced expression of glia maturation factor correlates with glial activation in the brain of triple transgenic Alzheimer's disease mice. Neurochem Res 37(7): 218-225.
- Luan K, Rosales JL, Lee KY (2013) Viewpoint: Crosstalks between neurofibrillary tangles and amyloid plaque formation. Ageing Res Rev 12(1): 174-181.
- Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, et al. (2002) Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 10(1): 24-35.
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, et al. (2001) MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging 22(5): 747-754.
- Ahmed ME, Iyer S, Thangavel R, Kempuraj D, Selvakumar GP, et al. (2017) Co-Localization of Glia Maturation Factor with NLRP3 Inflammasome and Autophagosome Markers in Human Alzheimer's Disease Brain. J Alzheimers Dis 60(3): 1143-1160.
- Thangavel R, Bhagavan SM, Ramaswamy SB, Surpur S, Govindarajan R, et al. (2018) Co Expression of Glia Maturation Factor and Apolipoprotein E4 in Alzheimer's Disease Brain. J Alzheimers Dis 61(2): 553-560.
- Zaheer A, Zaheer S, Thangavel R, Wu Y, Sahu SK, et al. (2008) Glia maturation factor modulates beta-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res 1208: 192-203.
- Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, et al. (2013) Glia maturation factor induces interleukin-33 release from astrocytes: implications for neurodegenerative diseases. J Neuroimmune Pharmacol 8(3): 643-650.
- Ahmed ME, Selvakumar GP, Kempuraj D, Thangavel R, Mentor S, et al. (2019) Synergy in Disruption of Mitochondrial Dynamics by A beta (1-42) and Glia Maturation Factor (GMF) in SHSY5Y Cells Is Mediated Through Alterations in Fission and Fusion Proteins. Mol Neurobiol 56(10): 6964-6975.






























