A Case Report of Uncommon Localization of Primary Malignant Extra-Dural and Intradural Extra-Medullary Melanoma of the 8th Cervical Spinal Nerve Root
Ait Bachir M*, Kechfoud H and Tliba S
Department of Neurosurgery, Nedir Mohamed Clinical Medical College of Tizi ouzou University, Algeria
Submission: October 09, 2019; Published: December 13, 2019
*Corresponding author: Ait Bachir M, Department of Neurosurgery, Nedir Mohamed Clinical Medical College of Tizi ouzou University, Algeria
How to cite this article: Ait Bachir M, Kechfoud H, Tliba S. A Case Report of Uncommon Localization of Primary Malignant Extra-Dural and Intradural Extra-Medullary Melanoma of the 8th Cervical Spinal Nerve Root. Open Access J Neurol Neurosurg. 2019; 12(3): 555838. DOI: 10.19080/OAJNN.2019.12.555838.
Abstract
Primary spinal melanoma is a rare lesion, which occurs throughout the cranial and spinal regions, however, is primarily observed in the cervical spine. we report on a case of an uncommon localization of primary malignant extra-dual and intradural extra-medullary melanoma of the 8th cervical spinal nerve root which developed in a 27-year man who complained of pain in the lower cervical spine, with left upper extremity tingling sensation. Neuro-radiological features of this extra-Dural mass were suggestive of a nerve sheath tumor. Magnetic Resonance Imaging (MRI) of cervical spine showed a lesion in extra-Dural and intradural extra-medullary mass who were suggestive of a nerve sheath tumor, but the histological examination confirmed the histological nature of primary malignant extra-Dural and intradural extra-medullary melanoma. The resection was complete using a standard posterior midline approach, which was followed by chemotherapy. and the course has been satisfactory after 06 months follow-up. Subsequent to the surgery, the patient was discharged with improved motor capacity and a follow up MRI scan showed no recurrence after six months. The present study demonstrates that it is critical for neurosurgeons to focus on increasing the accuracy of initial diagnoses in order to make informed decisions regarding the requirement for surgical resection. There are no evidence-based guidelines regarding primary spinal melanoma, for example specific incidence, treatment or prognosis guidelines.
Keywords: Primary malignant melanoma Spinal cord tumor Nerve root, Extra-Dural and intradural extra-medullary melanoma MRI
Abbrevations: MRI: Magnetic Resonance Imaging; CNS: Central Nervous System; HMB: Human Melanoma Black; EMA: Epithelial Membrane Antigen
Introduction
Primary malignant melanoma of the Central Nervous System (CNS) accounts for 1% of all cases of melanoma, it’s a highly malignant type of tumor, with a median survival rate of between six and 12 months, and a five-year survival rate of <10% [1-4]. Melanotic lesions of the spinal roots are rare, and may be intraor extradural, or may possess intra- and extramural components [5]. Spinal melanoma is primarily found in the middle or lower thoracic spine. Since the first case of spinal melanoma was reported by Hirschberg in 1906, about 100 cases have been reported in the literature [6]. Pigmented cystic lesions with characterless cyst capsule may be the result of extensive necrosis or cystic degeneration of either a melanoma or a melanotic schwannoma [1-6]. In the present case, the diagnosis of primaryspinal malignant melanoma was obtained through histological, and immunohistochemical analyses. The aim of the present case report is to discuss the diagnosis, treatment and prognosis of this rare condition.
Case Report
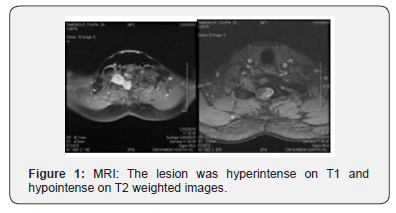
A 27-year-man was admitted to the Clinical Medical College of Bejaia in July December 2015 with history of low back pain in the lower cervical spine bilateral lower-extremity numbness and right upper extremity tingling sensation for past one week which was abrupt in onset and severe in nature. The pain was non-radiating and confined to low back on the bilateral side, affecting her activities. Upon admission to hospital, a neurologicalexamination revealed hypoesthesia below the T8 level and a neurological motor deficit of the bilateral lower extremity. There were no neurocutaneous markers. Magnetic Resonance Imaging (MRI) of cervical spine revealed a well-defined extra-spinal oval lesion in the Right C7-D1 neural foramen causing widening of the canal mimicking neurofibroma or schwannoma with a mass in intra- and extradural space compressing the spinal cord at this level and a large globular paraspinal mass. The lesion was hyperintense on T1 and hypointense on T2 weighted images with homogeneous contrast enhancement suggestive of spinal nerve sheath tumor (Figure 1).
Operative technique

A 27-year-man was admitted to the Clinical Medical College of Bejaia in July December 2015 with history of low back pain in the lower cervical spine bilateral lower-extremity numbness and right upper extremity tingling sensation for past one week which was abrupt in onset and severe in nature. The pain was non-radiating and confined to low back on the bilateral side, affecting her activities. Upon admission to hospital, a neurologicalexamination revealed hypoesthesia below the T8 level and a neurological motor deficit of the bilateral lower extremity. There were no neurocutaneous markers. Magnetic Resonance Imaging (MRI) of cervical spine revealed a well-defined extra-spinal oval lesion in the Right C7-D1 neural foramen causing widening of the canal mimicking neurofibroma or schwannoma with a mass in intra- and extradural space compressing the spinal cord at this level and a large globular paraspinal mass. The lesion was hyperintense on T1 and hypointense on T2 weighted images with homogeneous contrast enhancement suggestive of spinal nerve sheath tumor (Figure 1).
Histological Examination
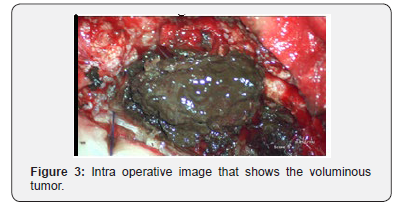
Standard histopathological examination of the tumor samples revealed cytologic atypia, mitotic activity and tumor cells with cytoplasmic deposition. These cellular characteristics were highly indicative of malignant melanoma. Immunohistochemically, the neoplastic cells stained positive for, S-100 protein and the malignant melanoma monoclonal antibody, Human Melanoma Black (HMB) 45. However, the neoplastic cells were negative for Epithelial Membrane Antigen (EMA) and Neuron-Specific Enolase (NSE. Thus, the histopathological and immunohistochemical findings indicate that the tumor originated from a melanocyte. Preoperative analyses, including, full body computed tomography, MRI, abdominal ultrasound and ocular examination, revealed no lesions in the patient’s other organs (Figure 4).
Immediate outcome
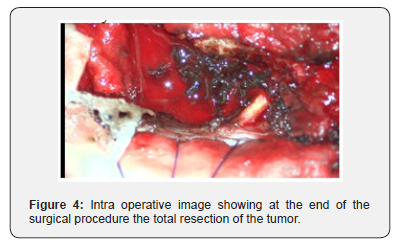
Post-operatively, patient was completely relieved of her low back pain and a postoperative clinical examination of the patient revealed decrease in motor strength. Additional dermatologic and ophthalmologic examinations did not reveal any other foci of primary melanoma. The patient was discharged with improvement of motor weakness. Repeated MRI scans of the cervico-thoracic spine conducted two weeks after surgery, demonstrated that the mass had been totally resected the patient was transferred to the oncology department a Subsequent radiotherapy and chemotherapy were administered [7-14].
Discussion
The approach of our study is similar to that reported from literature. we analyzed the literature we found that there has beenno widely agreed definition of ‘cure, after initial surgery due to the relative rarity of primary spinal melanoma .Various investigators have sought to define factors in patients that may be associated with later risk of relapse. Melanotic lesions are reported to have a more aggressive course when compared with their amelanotic counterpart [3,11,13]. Melanotic schwannoma, a variant of schwannoma, is characterized by the presence of spindle or epitheloid cell with pigmented granules [13]. Kasnatikul, in 1989, had reported a case of spinal melanotic arachnoid cyst, which was characterized by a melanotic cyst, lined with a cuboidal epithelium which was in continuation with the leptomeninges [9]. Cystic melanotic lesions reported are primarily either melanoma or schwannoma that have undergone cystic degeneration or hemorrhagic transformation [4,15-20]. These tumors are more often associated with neurocutaneous syndromes such as neurofibromatosis as opposed to this reported case, where there were no neurocutaneous stigmata. Surgical excision is the accepted treatment of choice in most cases, except for recurrent and malignant lesions [18]. These have higher risk of malignant transformation and metastasis; hence patients have to be followed-up periodically for recurrence of symptoms and also look out for other complaints suggestive of metastasis [19]. More than 90% of spinal melanomas metastasize and grow rapidly, which usually results in a fatal outcome within six months [12]. Among all of the published cases of primary spinal melanoma, including the present case, the patients have presented non-specific and progressive symptoms of myelopathy. These symptoms mimic those of other intraspinal mass lesions, which occupy similar locations and demonstrate similar growth patterns, including spinal meningioma, meningeal melanocytomas and metastatic melanoma.
Radiological study
The typical pattern of spinal melanoma observed using MRI, includes signal hyperintensity on T1-weighted images and signal is intensity or hypo intensity on T2-weighted images. These signal characteristics are inconsistent as the MRI signal depends on the presence of melanin, intratumorally hemorrhages and fat deposits, which complicates the majority of spinal melanoma images. MRI scanning aids diagnosis, however, it does not specifically differentiate between primary melanoma and other malignant lesions. However, T1-weighted images with hyperintensity and T2-weighted images with hypo intensity are typical features for melanoma, and atypical for meningioma. Therefore, it is difficult to exclude the diagnosis of spinal meningioma prior to surgery, as intratumorally bleeding may result in an uneven hyper intensive signal in T1-weighted images [21-23].
Histological examination
melanin-containing tumors, including melanocytotic, melanocytoma, malignant melanoma and meningeal melanomatous, exhibit spindle or epithelioid cells arranged in sheets, bundles, nests or whorls containing variable quantities of melanin pigment in the cytoplasm [8]. The differential diagnosisbetween malignant melanoma and meningeal melanocytoma require consideration, as the two originate from melanocytes. In the present case report, the presence of the histological characteristics of Positive staining of the anti-melanoma antibody, HMB45 and the S-100 protein confirmed the diagnosis of a malignant melanoma. Immunohistochemical analysis facilitates the differentiation between these different melanin-containing tumors. Positive staining of the anti-melanoma antibody, HMB45 and the S-100 protein indicates that cells are of melanocytic origin. A negative reaction for EMA eliminates the possibility of a mass being a melanotic meningioma of the spinal cord, and a negative reaction for EMA and NSE exclude metastatic carcinoma of melanocytic origin. Thus, in order to accurately diagnose primary spinal malignant melanoma, it is important to combine histological, radiological and immunohistochemical analyses. In the present case, complete surgical resection was recommended in order to obtain a curative outcome. Local control rates have been reported to be four-fold higher if complete resection is achieved [2]. Intraoperatively, the differentiation between various melanin-containing tumors is often difficult.
Therapeutic Adjuvant
The selection of an appropriate individual therapy remains controversial in the treatment of melanoma. Attitudes towards adjuvant treatment vary worldwide. While melanoma is a radiotherapy-resistant tumor, patients benefit from surgical resection, which has been reported to significantly alleviate the symptoms that result from its compressive effect. Surgical resection has also been reported to reduce the growth rate of melanoma [10]. Furthermore, Hamilton, et al. [7] reported preoperative radiotherapy in a patient with spinal melanoma and obtained satisfactory clinical outcomes. The radiotherapy dose depended on the tumor size, location, compression symptoms and patient tolerance; however, a dose of 12–24 Gy was recommended by the majority of the doctors. A previous study reported that Gamma Knife therapy improved the clinical outcome and reduced the complication rate in metastatic CNS melanoma [21,22]. However, the efficiency and long-term survival rate of Gamma Knife therapy requires further investigation to confirm these findings. The efficacy of radio- and chemotherapy remains controversial in the treatment of melanoma. A study in the USA reported that high-dose interferon treatment improved patient prognosis, although it resulted in severe side-effects [14]. A meta-analysis showed that chemo- and biological therapy were capable of reducing the recurrence rate and increasing survival by only 3% after five years [23]. The patient in the present case was treated with chemotherapy subsequent to surgery and no tumor recurrence was observed at the three-month follow-up.
Conclusion
The clinical features of primary malignant extra-Dural and intradural extra-medullary melanoma are complex and may be easily misdiagnosed as other spinal lesions. Primary malignantmelanoma is a particularly rare and aggressive tumor; therefore, total resection is recommended. An accurate diagnosis based on histological and immunohistochemical analyses of the resected tissue, is critical for selecting the appropriate therapy to enhance patient outcome. Thus, the present case report illustrates that primary spinal cord melanoma must be diagnosed with caution due to its variable clinical and radiologic presentation -the importance for neurosurgeons to analyze radiological data carefully to increase the accuracy of their initial diagnosis. The diagnostic potential of malignant melanoma requires consideration at the time of surgery to establish the need for aggressive surgical resection. Thus, early complete surgical resection followed by individualized radio or chemotherapy may enhance patient outcome. Despite treatment strategies involving total resection and adjuvant therapy, the prognosis of patients with primary spinal melanoma remains particularly poor.
References
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, et al. (2001) Final version of the American Joint Committee on cancer staging system for cutaneous melanoma. J Clin Oncol 19: 3635-3648.
- Bhatia S, Tykodi SS, Thompson JA (2009) Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 23(6): 488-496.
- Claessens N, Heymans O, Arrese JE, Garcia R, Oelbrandt B, et al. (2003) Cutaneous psammomatous melanotic schwannoma: non-recurrence with surgical excision. Am J Clin Dermatol 4(11): 799-802.
- Dublin AB, Norman D (1979) Fluid--fluid level in cystic cerebral metastatic melanoma. J Comput Assist Tomogr 3(5): 650-652.
- Ganiüsmen O, Özer FD, Mete M, Özdemir N, Bayol Ü (2012) Slow progression and benign course of a primary malign melanoma of a lumbar nerve root. Clin Neurol Neurosurg 114(2): 166-168.
- Fuld AD, Speck ME, Harris BT, Simmons NE, Corless CL, et al. (2011) Primary melanoma of the spinal cord: a case report, molecular footprint, and review of the literature. J Clin Oncol 29(17): e499-e502.
- Hamilton AJ, Lulu BA, Fosmire H, Stea B, Cassady JR (1995) Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery 36(2): 311-319.
- Hayward RD (1976) Malignant melanoma and the central nervous system. A guide for classification based on the clinical findings. J Neurol Neurosurg Psychiatry 39(6): 526-530.
- Kasantikul V, Shuangshoti S, Pattanaruenglai A, Kaoroptham S (1989) Intraspinal melanotic arachnoid cyst and lipoma in neurocutaneous melanosis. Surg Neurol 31(2): 138-141.
- Katalinic D, Anic B, Stern-Padovan R, Mayer M, Sentic M, et al. (2011) Low back pain as the presenting sign in a patient with primary extradural melanoma of the thoracic spine - a metastatic disease 17 years after complete surgical resection. World J Surg Oncol 9: 1502011.
- Kwon SC, Rhim SC, Lee DH, Roh SW, Kang SK (2004) Primary malignant melanoma of the cervical spinal nerve root. Yonsei Med J 45(2): 345-348.
- Lee CH, Moon KY, Chung CK, Kim HJ, Chang KH, et al. (1976) Primary intradural extramedullary melanoma of the cervical spinal cord: case report. Spine 35(8): E303-E307.
- Mandybur TI (1974) Melanotic nerve sheath tumors. J Neurosurg 41: 187-192.
- O Day SJ, Atkins MB, Boasberg P, Wang HJ, Thompson JA, et al. (2009) Phase II multicenter trial of maintenance biotherapy after induction concurrent Biochemotherapy for patients with metastatic melanoma. J Clin Oncol 27(36): 6207-6212.
- Parmar HA, Ibrahim M, Castillo M, Mukherji SK (2007) Pictorial essay: Diverse imaging features of spinal schwannomas. J Comput Assist Tomogr 31(3): 329-334.
- Reed H (1946) A congenital melanoma of lip with cystic degeneration. Br J Surg 34: 95.
- Santaguida C, Sabbagh AJ, Guiot MC, Del Maestro RF (2004) Aggressive intramedullary melanotic schwannoma: Case report. Neurosurgery 55(6): 1430.
- Scheithauer BW, Erdogan S, Rodriguez FJ, Burger PC, Woodruff JM, et al. (2009) Malignant peripheral nerve sheath tumors of cranial nerves and intracranial contents: A clinicopathologic study of 17 cases. Am J Surg Pathol 33(3): 325-338.
- Takahashi I, Sugimoto S, Nunomura M, Takahashi A, Aida T, et al. (1990) A case of cystic metastatic intracranial amelanotic melanoma--analysis of findings in CT and MRI. No to Shinkei 42(11): 1031-1034.
- Tipton A, Goldman SM, Fishman EK, Arnold P (1987) Cystic renal melanoma: CT/ultrasound correlation. Urol Radiol 9(1): 39-41.
- Yu J, Zhao DD, Chen S, Zhang JM, Xu J (2012) Primary melanoma of the cervical spine with cerebral metastases: case report and review of the literature. J Int Med Res 40(3): 1207-1215.
- Yu J, Zhao DD, Chen S, Zhang JM, Xu J, et al. (2012) Primary melanoma of the cervical spine with cerebral metastases: case report and review of the literature. J Int Med Res 40(3): 1207-1215.
- Wheatley K, Ives N, Eggermont A, (2007) Interferon-α as adjuvant therapy for melanoma: an individual patient data meta-analysis of randomized trials. J Clin Oncol 25: 8526.






























