Neurological Manifestations of Cobalamin Deficiency
Ammouri W¹*, Harmouche H¹, Khibri H¹, Benkirane S², Azlarab M², Mouatassim N³, Maamar M¹, Mezalek Tazi Z¹ and Adnaoui M¹
1Department of Internal medicine, University Mohamed V of medicine, Morocco
2Laboratoire central d’hématologie, University Mohamed V of medicine, Morocco
3Department of Radiology, University Mohamed V of medicine, Morocco
Submission: November 12, 2019; Published: December 05, 2019
*Corresponding author: Ammouri wafa, Department of Internal medicine, University Mohamed V of medicine, Unité d hématologie clinique, Ibn Sina Hospital, Rue Lamfadel Cherkaoui, BP 6527, Rabat, Morocco
How to cite this article: Ammouri W, Harmouche H, Khibri H, Benkirane S, Azlarab M, et al. Neurological Manifestations of Cobalamin Deficiency. Open Access J Neurol Neurosurg. 2019; 12(2): 555834. DOI: 10.19080/OAJNN.2019.12.555834.
Abstract
Vitamin B12 (cobalamin) is synthesized by microorganisms and detected in trace amounts mostly in foods of animal origin. The absorption and transporting mechanisms depended on three key proteins: haptocorrin, intrinsic factor and transcobalamin. On the peripherу cobalamin circulates only in binding with proteins transcobalamin I and II (holotranscobalamin). Holotranscobalamin is absorbed by different cells, whereas transcobalamin I-binded vitamin B12 - only by liver and kidneys. Two forms of cobalamin were identified as coenzymes of cellular reactions which are methylcobalamin and hydroxyadenosylcobalamin. The interaction between folate and B12 is responsible for the megaloblastic anemia seen in both vitamin deficiencies. Vitamin B 12 is necessary for the development and initial myelination of the central nervous system as well as for the maintenance of its normal function. Neurologic manifestations of B12 deficiency are polymorph. Causes are dominated by Pernicious anemia and B12 vitamin non dissociation that is frequent in the elderly. It is estimated that 40% of older adults have vitamin B12 deficiencies and most due to cobalamin malabsorption. Neurologic and psychiatic manifestations of B12 deficiency are rarely initial symptoms. They are usually attributed to the intervention of vitamin B12 in the isomerization reaction of Methylmalonic Acid (MMA) to succinic acid. Neurological involvement often occurs along with macrocytic anemia but can arise in the absence of either anemia or macrocytosis. Also, it is unclear why vitamin B12 deficiency leads to neurological disease in some patients and hematological disease in others. The well-known major manifestations described include peripheral neuropathy, subacute combined degeneration of spinal cord, dementia, optic atrophy, psychosis and mood disturbance. Other, neurological disorders also described are cerebellar ataxia, abnormalities of cranial nerves, Parkinsonian syndrome and movement disorders. Substitutive vitamin therapy is required when diagnosis is strongly suspected, and etiologic assessment is negative. High-dose oral vitamin B12 tablets (1000 to 2000 μg) taken daily are as effective as intramuscular monthly injections in correcting blood and neurologic abnormalities.
Keywords: Cobalamin deficiency Neuropsychiatric disorders Vitamin Treatment Pernicious anemia management Cognition
Abbrevations: MMA: Methylmalonic Acid; HC: Haptocorrin; IF: Intrinsic Factor; TC: Transcobalamin; THF: Tetrahydrofolate; SAM: S-Adenosyl Methionine; HHCY: Hyperhomocysteinemia; CVD: Cardiovascular Disease; PA: Pernicious Anaemia; PCA: Parietal Cells; TC: Transcobalamin; RBC: Red Blood Cells; TMA: Thrombotic Microangiopathy
Introduction
The term vitamin B12 is used ubiquitously to refer to the different forms of cobalamin, such as Me-Cbl (Methylcobalamin-Me-Cbl) and Ado-Cbl (Cyanocobalamin Cn-Cbl), and they are also referred to as complete corrinoids. Me-Cbl and Ado-Cbl are the active forms of vitamins used as coenzymes in the cell [1]. Vitamin B12 is required in these coenzyme forms for the conversion of L-methylmalonyl CoA to succinyl-CoA and homocysteine to mehionine. Vitamin B12 is vital for appropriate red blood cell formation, neurological function, and DNA and RNA synthesis. Its dysfunction creates a shortage, affecting DNA synthesis and the physiological processes such as hematopoietic process of the erythrocytes [2]. The main causes of cobalamin deficiency are Pernicious anemia and B12 vitamin non dissociation that is frequent in the elderly [3]. Inaugural neurologic manifestations ofB12 deficiency arrays are rare and various thus making diagnosis difficult. Neurological involvement includes mainly combined spinal sclerosis, peripheral neuropathy and dementia [4-7]. Cerebellar ataxia and movement disorders are reported less often. Severity of neuropsychiatric features and therapeutic efficacy depends on the duration of signs and level of B12 deficiency. Also, though controversial, there has been recent concern that low Cbl levels in the elderly might cause nervous system damage, but studies specifically in the elderly have not consistently demonstrated improvements in neurologic function following therapy.
Macrocytic anemia may lack. Neuropsychiatric manifestations could be isolated or be the first manifestation of vitamin deficiency and occur without any hematological or gastrointestinal context [7,8]. Normal or decreased total plasma cobalamin level could not a reliable marker of vitamin deficiency. In difficult cases the content of holotranscobalamin, methylmalonic acid/homocysteine, and folate in the blood serum should be investigated besides carefully analysis of clinical manifestations [8]. The neurological complications may be reversed only if replacement treatment is administered early after onset. The review discusses the steps of vitamin B12 metabolism, its role in maintaining of neurological functions, the neurologic aspects and the management of vitamin B12 deficiency.
Physiological roles of vitamin B12
Vitamin B12 or cobalamin is an hydrosoluble vitamin synthesized by microorganisms and detected in trace amounts mostly in foods of animal origin. The recommended dietary allowance of Cbl for adults is 2.4 mg/day. It is estimated that only 50% of dietary vitamin B12 is absorbed by healthy adults. The absorption and transporting mechanisms depended on three key proteins haptocorrin, intrinsic factor and transcobalamin. In the stomach, the vitamin B12 released from food protein by peptic action is bound to Haptocorrin (HC) and travels to the duodenum, where pancreatic proteases digest the HC, releasing the vitamin B12 to bind to Intrinsic Factor (IF). The IF-vitamin B12 complex binds to a specific receptor called cubilin in the distal ileum and is internalized, eventually released from lysosomes, and transported into the blood. Both HC and Transcobalamin (TC) bind vitamin B12 in the circulation, although the latter is the cellular delivery protein. It is transported together with transcobalamin I, II or III to finally be stored in the hepatocyte [8]. Cobalamin act as a fundamental enzymatic cofactor in myelopoiesis (role in nucleotide synthesis) and in the synthesis of myelin of the central and peripheral nervous system. Theses functions are explained by the role of vitamin B12 as a cofactor for two enzymes methionine synthase and l-methylmalonyl-coenzyme A mutase. Thus, Cobalamin serves as a cofactor for methionine synthesis by transfer of methyl group to homocysteine which is an atherogenic and potential endothelial toxin. This conversion of homocysteine to methionine forms demethylated Tetrahydrofolate (THF) which is required for DNA synthesis. Further metabolism of methionineto S-Adenosyl Methionine (SAM) is essential for myelin synthesis and maintenance of neuronal integrity as well as neurotransmitter regulation. Disruptions in theses pathways produce elevated levels of homocysteine and methylmalonic acid, respectively. Homocysteine is neurotoxic through overstimulation of the N-methyl-D-aspartate receptors, and toxic to the vasculature because of activation of the coagulation system and adverse effect on the vascular endothelium. A lack of cobalamin leads to either destruction of myelin sheaths or the incorporation of abnormal fatty acids into myelin sheaths, thereby leading to impaired neural function and/or transmissions. This may be the underlying cause of the neurological symptoms seen in vitamin B12 deficiency
The resulting anemia may be macrocytic with bone marrow promegaloblastosis, reflecting ineffective erythropoiesis, or normocytic reflecting concomitant iron deficiency from achlorhydria. Dyssynchrony between the maturation of cytoplasm and that of nuclei leads to macrocytosis, immature nuclei, and hyper segmentation in granulocytes in the peripheral blood. The ineffective erythropoiesis results in intramedullary hemolysis and release of lactate dehydrogenase [9,10]. Inhibition of DNA synthesis due to vitamin B12 deficiency causes megaloblastic changes not only in bone marrow but also in other rapidly dividing cells, such as gastrointestinal epithelium explaining gastrointestinal disorders in patients with B12 deficiency.
Vitamin B12 and hyperhomocysteinemia
Actually, Hyperhomocysteinemia (HHcy) has been considered as a risk factor for systemic atherosclerosis and Cardiovascular Disease (CVD) and HHcy has been reported in many neurologic disorders including cognitive impairment and stroke, ischemic or hemorrhagic, and intracranial hemorrhage independent of longrecognized factors such as hyperlipidemia, hypertension, diabetes mellitus, and smoking. Homocysteine is synthesized by two metabolic pathways remethylation and transsulfuration. These pathways require vitamin B12 and folate for methionine synthesis and pyridoxal-5 phosphate for cystathionine synthesis [11-13]. B-vitamins appear to be more effective in lowering homocysteine (Hcy) in those with lower vitamin concentrations and elevated Hcy. HHcy is typically defined as levels >15 micromol/L. Homocysteine is due to genetic and acquired factors [14] (poor diet in folate and vitamin B12, older age, renal impairment, thyroid diseases, and malignancies), induced by the intake and the concentrations of vitamin B9 or B12 in the majority of cases. However, the most common cause of vitamin B12 deficiency with hyperhomocysteinemia is pernicious anemia [12,13]. Hyperhomcysteinemia (HHcy) is associated with vascular toxicity. For instance, Hcy interferes with the production of Nitric Oxide (NO), a gaseous master regulator of endothelial homeostasis. Also, HHcy induces loss of critical endothelial antioxidant systems and increases the intracellular concentration of reactive oxygen species yielding oxidative stress. The consequence is the disturb lipoprotein metabolism, contributing to the growth of atherosclerotic vascular lesions. Excess Hcy maybe indirectlyincorporated into proteins, a process referred to as protein N-homocysteinylation, inducing vascular damage. Lastly, cellular hypomethylation caused by build-up of S-Adenosylhomocysteine (AdoHcy) also contributes to the molecular basis of Hcy-induced vascular toxicity [14]. Treatment of hyperhomocysteinemia with folic acid and B vitamins seems to be effective in the prevention of the development of atherosclerosis, CVD, and strokes. However, data from literature show controversial results regarding the significance of homocysteine as a risk factor for CVD and stroke. Use of vitamin B12 in patients with elevated serum homocysteine levels and cardiovascular disease does not reduce the risk of myocardial infarction or stroke, or alter cognitive decline [15,16]. Causes of cobalamin deficiency (Table 1).
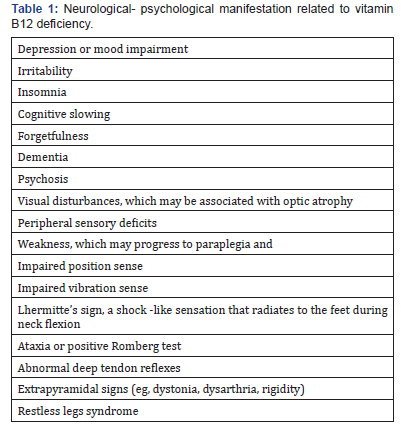
Table 1 list causes of vitamin B12 deficiency. Pernicious anemia is discussed below, since this is the most common cause of severe vitamin B deficiency. Vitamin B12 (Cbl) deficiency is silent and under-diagnosed, as its onset and progression are slow, and patients may get used to their symptoms.
The following are causes for cobalamin deficiency
a. Food-cobalamin malabsorption: Cbl deficiency is particularly common in the elderly with a prevalence ranging from 15%-40% [3,8,12].
b. It is most likely due to the high incidence of atrophic gastritis and associated achlorhydria-induced food-Cbl malabsorption. Food-cobalamin malabsorption (syndrome of non-dissociation of vitamin B12 from its carrier proteins), is characterized by an inability to release vitamin B12 from ingested food and/or from intestinal transport proteinsparticularly in the presence of hypochlorhydria in which absorption of unbound vitamin B12 is normal. Other causes of Cbl deficiency (e.g. Helicobacter pylori infection, antiacid therapy, use of metformin) may coexis.
c.Intestinal malabsorption
a decreased absorption of cobalamin is reported in cases of gastrectomy, bariatric surgery, Crohn’s disease, celiac disease, amyloidosis, scleroderma, intestinal lymphomas or tuberculosis, pancreatic insufficiency, bacterial overgrowth or fish tapeworm infection.
d. Autoimmune disease
Many patients with clinically expressed Cbl deficiency have IF-related malabsorption such as that seen in pernicious anemia. Pernicious Anaemia (PA) also known as Biermer’s disease [17] and Addisonian anemia is the most frequent cause of severe vitamin B12 deficiency worldwide, due to autoimmune atrophic gastritis, historically called pernicious anemia a macrocytic anemia due to vitamin B12 deficiency, which, in turn, is the result of deficiency of intrinsic factor. Patients with PA have been shown to have two types of antibodies, one to Parietal Cells (PCA) and the other to Intrinsic Factor (IFA) or its binding site in the small bowel. The immune response is directed against the gastric H/K– ATPase, which accounts for associated achlorhydria. This proton pump is responsible for acid secretion in the stomach and is the major protein of the secretory canaliculi of gastric parietal cells. Whether the stomach pathogen Helicobacter pylori plays a causative role in pernicious anemia is unclear [18]. However, iron deficiency anemia, a known complication of achlorhydria, occurs predominantly in women and precedes the onset of cobalamin-deficient pernicious anemia by about 20 years [17]. So, the patients with unexplained iron deficiency anemia should be checked for autoimmune gastritis and pernicious anemia. The incidence of PA increases with age and the mean age of patients with PA ranges from 59 to 62 years. The symptomatology is dominated by a profound megaloblastictype anemia and, in the most serious cases, by neurological alterations, which can precede the diagnosis of gastric atrophy by several decades. Also, Pernicious anemia correlates with other autoimmune disease (thyroiditis, vitiligo and type 1 diabetes mellitus) and as well as a genetic disease [19,20]. Patients with PA may also be at a higher risk for developing gastric cancer (adenocarcinoma and gastric carcinoid type Ⅰ) as an end-stage evolution of atrophic Gastritis (GA). An increased RR of biliary tract cancers and hematological malignancies was observed too [21]. Other causes of B12 deficiency include, low vitamin B12 intake, aside from strict vegans and newborns of vegan women. Vitamin B12 malabsorption syndromes comprise the genetic defects of proteins involved in vitamin B12 metabolism such as IF defiiency/ defects or transcobalamin II defiiency/defects.
e . Drugs
Metformin, antiacids, H2-blockers, proton-pump inhibitors, colchicine, anti convulsivants, antibiotics and nitrous oxide (N2O) [3,4,5]. Drugs may reduce cobalamin absorption through different mechanisms.
Inhibition of IF and acid secretion and trans enterocytic transport of cobalamin, respectively. N2O irreversibly oxidizes the cobalt core of Cbl and renders methyl Cbl inactive. Clinical manifestations of Cbl deficiency appear relatively rapidly with N2O toxicity because the metabolism is blocked at the cellular level. Postoperative neurologic dysfunction can be seen with N2O exposure during routine anesthesia if subclinical Cbl deficiency is present [22]. Nitrous oxide anesthesia may precipitate neurologic disease in people with unrecognized deficiency of vitamin B1.
The clinical manifestations of vitamin B12 deficiency
Vitamin B12 deficiency is silent and under-diagnosed, as its onset and progression are slow, and patients may get used to their symptoms. Nevertheless, the clinical consequences of undiagnosed vitamin B12 deficiency may be serious, including wide range of neurological and mood disorders. Patients usually exhibit symptoms of anemia with pallor, fatigue, lightheadedness, or tachycardia and decreased mental concentration. Involvement of small-bowel epithelium may result in malabsorption and diarrhea with weight loss. Anorexia is an additional common complaint. Glossitis is a frequent sign of megaloblastic anemia, with the patient displaying a painful, smooth, red tongue. Other symptoms are reported such as dyspeptic symptoms, epigastric discomfort, postprandial bloating and fullness, and earlysatiety. Skin hyperpigmentation and infertility have also been reported. The elevation in bilirubin levels, caused by ineffective erythropoiesis, manifests as jaundice. Patients may develop neurological symptoms [12,13].
Neurological abnormalities
Neurologic abnormalities are seen in 4-50% of vitamin B12 deficiency. It could be isolated or be the first manifestation of vitamin deficiency and occur without any hematological or gastrointestinal context. Vitamin B12 deficiency may affect both the central (brain, spinal cord and optic nerve) and the peripheral (peripheral nerves) nervous system. Demyelination is the initial finding, which progresses to axonal degeneration and neuronal death if left untreated. Neuropsychiatric manifestations associated with vitamin B12 deficiency include motor, sensory and autonomic symptoms, cognitive impairement, mood and psychotic symptoms. The most common neurologic findings in vitamin B12 deficiency are symmetric paresthesias (peripheral neuropathy seen in 25% of patients with vitamin B12 deficiency) or numbness and gait problems. The neuropathy is typically symmetrical and often affects the legs more than the arms. Some patients may also have subclinical involvement upon electrophysiological testing.
The classic sub-acute combined degeneration of the dorsal (posterior) and lateral columns is characteristic if present but may not occur in all cases. The neurologic features typically include a spastic paraparesis, extensor plantar response, and impaired perception of position and vibration. The appearance of motor symptoms is indicative of subacute combined degeneration involving the dorsal and lateral spinal columns.
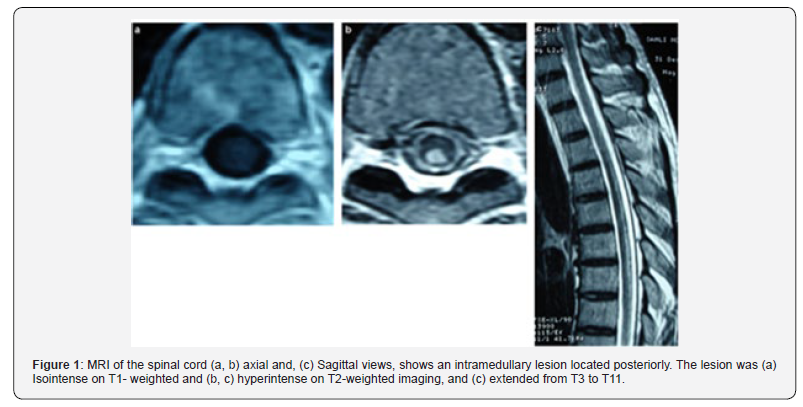
MRI has been considered the exam of choice (Figure 1) for detecting B12 deficiency-related central nervous system involvement and for excluding possible mimics. Imaging of the spinal cord in cases of severe myelopathy that are notinitially recognized as the result of vitamin B12 deficiency, had characteristic hyperintensity on T2-weighted imaging, described as an inverted V-shaped pattern in the cervical and thoracic spinal cord [6]. Brain involvement has been reported in B12 deficiency. Vitamin B12 deficiency has been also associated with attention deficits, acute mental-status and acute cognitive changes, with electroencephalography abnormalities. In an individual patient with dementia and Cbl deficiency, the response of the cognitive complaints to Cbl administration is variable and may relate to duration of deficiency. This relationship between cognitive decline or cognitive deficits and Cbl deficienc has been studied not only with vitamin B12 levels but also with HomoCysteine (Hcy) or MMA levels, holo Transcobalamin (TC) levels, and vitamin B12 intake. Observational studies have shown that high total Hcy increases the risk of cognitive impairment with or without dementia. HHcy cause agonism of N-methyl-D-aspartic acid receptors, leading to DNA damage and apoptosis, inhibition of hippocampal neurogenesis, decreased of gamma-amino-butyric acid-mediated inhibitory function and endothelial cell toxicity [23,24].
Low levels of vitamin B12 have been associated with neurocognitive disorders. This evidence-based analysis assessed the usefulness of serum vitamin B12 testing as it relates to brain function. Based on low to moderate quality of evidence, treatment with vitamin B12 and folate in patients who have mild cognitive impairment seems to slow the rate of brain atrophy compared with patients who have mild cognitive impairment receiving a placebo. Whether this translates into clinical benefit is unknown [22]. A recent metanalyses conducted by Ford AH [25,26], showed that raised total plasma homocysteine is associated with an increased risk of cognitive impairment and dementia, although available evidence from randomized controlled trials shows no obvious cognitive benefit of lowering homocysteine using B vitamins [27,28]. However, assessing vitamin B12 levels in patients with cognitive impairement or as part of work up of dementia is recommended. Radiologic aspects of low vitamin B12 or Hc include periventricular leukoencephalopathy, manifested as white matter hypodensity on CT scan or hyperintensity on T2 weighted MRI. A brain atrophy and silent brain infarcts. Other, neurological disorders also described such as cerebellar ataxia, abnormalities of cranial nerves, Parkinsonian syndrome, mood disturbance (depression and mania) and movement disorders [4,6,9].
A minority of patients exhibit mental or psychiatric disturbances (psychosis) or autonomic signs (bladder and erectile dysfunction). Optic neuropathy due to vitamin B12 deficiency occurs occasionally in adult patients. It is characterized by symmetric, painless and progressive visual loss. Central and centrocecal scotomas are the main ophthalmologic findings. Epilepsy is rarely seen in adult cases [4,5]. Neuropsychiatric symptoms can be present even in the absence of anemia or macrocytosis, and in some patients with a low-normal Cbl level[4]. The lack of these hematologic changes cannot be used to exclude vitamin B12 deficiency. Other neurological-psychological manifestations are listed in Table 2. The pediatric cases, makes note of involuntary movements and severe neurologic findings including hypotonia and developmental regression with delayed myelination and cerebral atrophy [29]. Maternal vitamin B12 deficiency during pregnancy or while breastfeeding may lead to neural tube defects, developmental delay, failure to thrive, hypotonia, ataxia, and anemia. Nutritional vitamin B12 deficiency in children, and a late diagnosis of this condition, may lead to irreversible neurologic damage such as growth and motor retardation and even convulsions. It is particularly important to recognize neurologic symptoms early, because the neurological lesions may not be reversed after replacement therapy with vitamin B12 [30].
Diagnosis of cobalamin deficiency
The recommended laboratory evaluation for patients with suspected vitamin B12 deficiency includes a complete blood count and serum vitamin B12 level. The diagnosis of PA relies on demonstration of megaloblastic anemia, low serum vitamin B12 levels, gastric atrophia, and the presence of antibodies to gastric parietal cell or intrinsic factor. The anemia is macrocytic and normochromic with reduction in the absolute number of reticulocytes. The patient’s Red Blood Cells (RBCs) exhibit marked anis poikilocytosis and numerous oval macrocytes. Although the hyper segmented neutrophils support the diagnosis of megaloblastic anemia, they are not specific. The variation in red-cell size and shape could lead to a misdiagnosis of microangiopathic hemolytic anemia instead of megaloblastic anemia. Also, macrocytosis is absent in 30% of PA patients if iron deficiency is associated. Moreover, masking of macrocytosis by α-thalassemia in PA patients with African origins who might present with microcytosis. Pancytopenia is often present with rates ranging from 5 to 37% [31-34].
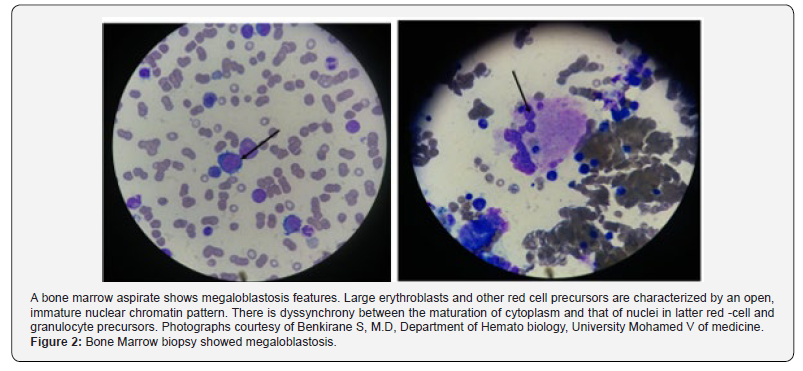
Schishocytes may be seen in megaloblastic anemias, as a result of erythroblast cytoskeletal fragility, reflecting the severity of dyserythropoiesis. Several cases of PA presenting with pseudo- Thrombotic Microangiopathy (TMA) are reported in the literature and treated with plasma transfusions or/and exchange. These cases are characterized by hemolysis, thrombocytopenia, and schistocytosis with higher mean LDH levels [34]. Very high LDH levels, mild-moderate thrombocytopenia, and a low reticulocyte count are strongly suggestive of pseudo-TMA and should prompt the physician to screen for cobalamin deficiency [31-35]. Bone marrow biopsy and aspiration are not necessary for the diagnosis of megaloblastic anemia (Figure 1 & 2) and may be misleading in cases of severe pancytopenia with hypercellularity, increased erythroblasts, and even cytogenetic abnormalities, confusing the diagnosis with acute leukemia. It shows a hypercellular bone marrow with a shift toward immaturity and abnormal maturation of erythroid and myeloid cell lines. The immature neutrophil seriesexhibits nuclear-cytoplasmic asynchrony with numerous giant metamyelocytes. The ineffective erythropoiesis and myelopoiesis are responsible for the pancytopenia in megaloblastic anemia, despite marrow hypercellularity [3,4].
Serum vitamin B12 and serum folate levels should be determined concurrently to correctly identify patients deficient in either or both. But it has limited sensitivity and specificity. Some authors are in support of vitamin B12 deficiency screening in the elderly. But, no formal recommendation for screening in asymptomatic people exist, the higher risk of occurrence in the elderly and easy and safe replacement therapy, more liberal testing and treatment is advised in the elderly. Differentiating between vitamin B12 deficiency and folate deficiency is essential to patient management, because treatment of vitamin B12 deficient patients with folate alone may reverse the megaloblastic blood picture, but the associated neurologic damage may worsen. Cobalamin level is measured by automated competitive-binding immunoenzymatic binding luminescence method
The results of which may not always accurately reflect actual vitamin B12 stores. Low level (<100 pg per millilite) is usually associated with clinical deficiency. But, Both false negative and false positive values are common (occurring in up to 50% of tests) due to the fact that only 20% of the total measured vitamin B 12 is on the cellular delivery protein, Transcobalamin (TC); the remainder is bound to haptocorrin, a protein of unknown function. IF antibodies may bind the test IF reagent and if there is a failure in the denaturation step intended to denature IF-blocking antibodies, spuriously normal or increased vitamin B12 levels can be measured [31,32]. In this situation, Measurement of Serum Methylmalonic Acid and total Homocysteine is useful in making the diagnosis of vitamin B 12 (markedly elevated levels) deficiency in patients who have not received treatment including those who have only neurologic manifestations of deficiency. Elevated levels of Total Homocysteine (THcy) and Methylmalonic Acid (MMA)have been proven as markers for insufficient intracellular vitamin B12. Also, the levels can be remeasured to document adequate vitamin B12 replacement. An elevated level of methylmalonic acid is reasonably specific for vitamin B12 deficiency, and the level always decreases with vitamin B12 therapy. If MMA concentrations are elevated, it is recommended to rule out other possible causes of elevated MMA, including renal insufficiency or intravascular volume depletion.
The level of serum total homocysteine is less specific, since it is also elevated in folate deficiency classic homocystinuria, and renal failure. Because the only fraction of dietary vitamin B12 bioavailable for systemic distribution is in the form of holoTC, the level of holoTC in serum has been successfully utilized as a surrogate of bioactive vitamin B12. This marker is considered to be more accurate in assessing the biologically active fraction of vitamin B12 in circulation than serum vitamin B12 itself, and its level correlates with the concentration of serum vitamin B12 in erythrocytes [33]. It is unknown whether holoTC levels vary in patients harboring inborn errors affecting intracellular vitamin B12 metabolism. Thus, the diagnostic value of holoTC as a first line test still needs further investigation. A deficit of intrinsic factor may be demonstrated using the Schilling test, a dynamic multistep investigatory test which involves the ingestion of isotope-labeled vitamin B12, followed by an injection of unlabeled vitamin B12. Given its complexity and problems related to the use of radioactive agents, the schilling test is now being replaced by other diagnostic strategies such as the detection of intrinsic factor antibodies
Patients diagnosed with vitamin B12 deficiency whose history and physical examination do not suggest an obvious dietary ormalabsorptive etiology should be tested for pernicious anemia. A positive test for anti–Intrinsic Factor (IFA) or anti-parietal-cell antibodies (by immunoblotting, ELISA and chemiluminescent immunoassay methods) identifies an autoimmune basis for the gastric atrophy in pernicious anemia. Anti-parietal cell antibodies are found in 90% of patients with PA but have low specificity and are seen in atrophic gastritis without megaloblastic anemia as well as in various autoimmune disorders. IFA are less sensitive, being found in only 60% of patients with PA, but are considered highly specific for PA. However, a positive correlation between the increasing histological score of body mucosa atrophy and the titer of both antibodies can be observed [35]. Surveillance for autoimmune thyroid disease is reasonable in patients with positive antibody tests. A diagnostic workup of megaloblastic anemia also should include evaluation of iron, because the bone marrow is overloaded with iron that cannot be utilized during the megaloblastic state.
Therefore, iron supplementation may be warranted even though the patient has an initial normal serum iron value . Chronic atrophic gastritis can be diagnosed on the basis of an elevated fasting serum gastrin level and a low level of serum pepsinogen I. Some experts recommend endoscopy to confirm gastritis and rule out gastric carcinoid and other gastric cancers, since patients with pernicious anemia are at increased risk for such cancers. In PA patients, the mucosa of the cardia and fundus is thinned and atrophied, with shrunken glands and containing few principal and parietal cells, while usually the mucosa of the antrum is spared. However, a concomitant antral atrophic gastritis may be observed in 25% of PA patients [36,37]. These data strongly suggest that an extension of gastritis to the gastric antrum does not necessarily exclude the diagnosis of PA and the presence of gastric autoimmunity.
Management
The therapeutic recommendations with regard to dosage and administration of vitamin B12 substitution treatment are divergent. Vitamin B12 must be administered parenterally. Patients generally receive an intramuscular injection of 1000 mcg B12 every day or every other day during the first week of treatment. The next month, they receive injections every week, subsequently followed by monthly injections. The alternative to intramuscular injection B12 is high-dose oral B12, a 1000 to 2000 mcg/day has been demonstrated to be effective [38-42]. However, despite many studies suggesting oral administration of vitamin B12 to be easy, effective and less costly than intramuscular administration, debate surrounds the effectiveness of the oral route. Patients should be offered this alternative after an informed discussion on the advantages and disadvantages of both treatment options.
The effect of oral cobalamin treatment in patients presenting with severe neurological manifestations has not yet been adequately documented. Although recommendations are to always use the parenteral route in severe neurological manifestations.Approved sublingual and intranasal formulations of B12 are also available [39]. Absorption rates improve with supplementation. Patients older than 50 years and vegans or strict vegetarians should consume foods fortified with vitamin B12 or take vitamin B12 supplements. Women at high risk or with known deficiency should supplement with vitamin B12 during pregnancy or while breastfeeding. Patients with PA have a higher frequency of thyroid disease, diabetes, stomach cancer, and iron deficiency and should be screened for these conditions [37,40]. Once treatment has been initiated, obtain repeat plasma vitamin B12, MMA and Hcy levels in 4-6 weeks to assess response to treatment. Monitoring of vitamin B12 levels should be performed every 6 to 12 months. Follow-up MR imaging findings correlate with clinical outcome after treatment with vitamin B12 supplementation. The abnormal MR signals on the spinal cord might either disappear on follow-up after months, or sometimes, it might persist, especially in cases diagnosed and treated at an advanced stage [43,44]. However, the improvement or reversal of neuropsychiatric symptoms varies according to symptom severity, duration and clinical diagnosis
Conclusion
B12 deficiency is often overlooked and a common cause of megaloblastic anemia and various neuropsychiatric symptoms. Neuropsychiatric symptoms may precede hematologic signs and are represented by myelopathy, neuropathy, dementia and, less often, optic nerve atrophy. Laboratory assessment should include a complete blood count and serum vitamin B12 level. Measurement of homocysteine and serum methylmalonic acid should be used to confirm deficiency in asymptomatic high-risk patients with low-normal levels of vitamin B12. Early diagnosis and treatment of vitamin B12 deficiency, can prevent irreversible neurological damage and reduce morbidity among patients.
References
- Hodgkin DC, Kamper J, Mackay M, Pickworth J, Trueblood KN, et al. (1956) Structure of Vitamin B12. Nature 178(4524): 64-66.
- Randaccio L, Geremia S, Demitri N, Wuerges J (2010) Vitamin B12: unique metalorganic compounds and the most complex vitamins. Molecules 15(5): 3228-3259.
- Pennypacker LC, Allen RH, Kelly JP, Matthews LM, Grigsby J, et al. (1992) High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc 40: 1197-204.
- Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, et al. (1988) Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 318(26): 1720-1728.
- Ammouri W, Harmouche H, Maamar M, Mezalek Tazi Z, Adnaoui M (2016) Seizures and psychiatric manifestations heralding Biermer's disease. Rev Neurol (Paris) 172(6-7): 404-405.
- Hemmer B, Glocker FX, Schumacher M, Deuschl G, Lücking CH (1998) Subacute combined degeneration: clinical, electrophysiological, and magnetic resonance imaging finding. J Neurol Neurosurg Psychiatry 65(6): 822-827.
- Maamar M, Tazi-Mezalek Z, Harmouche H, Ammouri W, Zahlane M, et al. (2006) Neurological manifestations of vitamin B12 deficiency: a retrospective study of 26 cases. Rev Med Interne 27(6): 442-447.
- Green R (2017) Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. 129(19): 2603-2611.
- Khan U, Crossley C, Kalra L (2008) Homocysteine and its relationship to stroke subtypes in a UK black population: The South London Ethnicity and Stroke Study. Stroke 39(11): 2943-2949.
- Selhub J, Bagley LC, Miller J, Rosenberg IH (2000) B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr 71(2): 614S-620S.
- Moretti R, Caruso P (2019) The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. Int J Mol Sci 20(1).
- Stabler SP (2013) Clinical practice. Vitamin B12 deficiency. N Engl J Med 368: 149-160.
- Wintrobe MM, Lee GR, Boggs DR, Bithell TC, Foerster J (1981) Megaloblastic and nonmegaloblastic macrocytic anemias. In: Wintrobe MM, Lee GR, et al. (Eds.), Clinical hematology. (8th ),Philadelphia: Lea & Febiger, USA, pp. 559-604.
- Kang SS, Wong PWK (1994) Genetic and nongenetic factors for moderate hyperhomocysteinemia. Atherosclerosis 119(2): 135-138.
- Rafnsson SB, Saravanan P, Bhopal RS, Yajnik CS (2011) Is a low blood level of vitamin B12 a cardiovascular and diabetes risk factor? A systematic review of cohort studies. Eur J Nutr 50(2): 97-106.
- Spence JD (2016) Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Spence JD. Nutr Res 36(2): 109-116.
- Toh BH, Whittingham S, Alderuccio F (2006) Gastritis and pernicious anemia. Autoimmune Dis 39: 527-546.
- Saito M, Morioka M, Wakasa K, Izumiyama K, Mori A, et al. (2013) In Japanese patients with type A gastritis with pernicious anemia the condition is very poorly associated with Helicobacter pylori infection. J Infect Chemother 19(2): 208-210.
- Petite J, Rosset N, Chapuis B, Jeannet M (1987) Genetic factors predisposing to autoimmune diseases. Study of HLA antigens in a family with pernicious anemia and thyroid diseases. Schweiz Med Wochenschr 117(50): 2032-2027.
- Zulfiqar AA, Andres E (2017) Association pernicious anemia and autoimmune polyendocrinopathy: a retrospective study. J Med Life 10(4): 250-253.
- Vannella L, Lahner E, Osborn J, Annibale B (2013) Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 37(4): 375-382.
- Egan W, Steinberg E, Rose J (2018) Vitamin B12 deficiency-induced neuropathy secondary to prolonged recreational use of nitrous oxide. Am J Emerg Med 36(9): 1717.e1-1717.e2.
- Scalabrino G (2009) The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog Neurobiol 88(3): 203-220.
- Scalabrino G, Veber D, Tredici G (2014) Relationships between cobalamin, epidermal growth factor, and normal prions in the myelin maintenance of central nervous system. Int J Biochem Cell Biol 55: 232-241.
- Green R, Datta Mitra A (2017) Megaloblastic anemias: nutritional and other causes. Med Clin North Am 101(2): 297-317.
- Ford AH, Almeida OP (2012) Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J Alzheimers Dis 29(1): 133-149.
- Flicker L, Vasikaran SD, Thomas J, Acres JM, Norman P, et al. (2006) Efficacy of B vitamins in lowering homocysteine in older men: Maximal effects for those with B12 deficiency and hyperhomocysteinemia. Stroke 37(2): 547-549.
- Ford AH, Almeida OP (2019) Effect of Vitamin B Supplementation on Cognitive Function in the Elderly: A Systematic Review and Meta-Analysis. Drugs Aging 36(5): 419-434.
- Avci Z, Turul T, Aysun S, Unal I (2003) Involuntary movements and magnetic resonance imaging findings in infantile cobalamine (vitamin B12) deficiency. Pediatrics 112(3pt 1): 684-686.
- Andres E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB, et al. (2004) Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 171(3): 251-259.
- Shishido T, Hiroshima Y, Uematsu N, Kazumoto H, Kaiume H, et al. (2018) Successful treatment with mecobalamin in a pernicious anemia patient presenting with false-normal serum vitamin B12. Rinsho Ketsueki 59(6): 675-681.
- Tavares J, Baptista B, Gonçalves B, Horta AB (2019) Pernicious Anaemia with Normal Vitamin B12. Eur J Case Rep Intern Med 6(2): 001045.
- Valente E, Scott JM, Ueland PM, Cunningham C, Casey M, et al. (2011) Diagnostic accuracy of holotranscobalamin, methylmalonicacid, serum cobalamin, and other indicators of tissue vitamin B12 status in the elderly. Clin Chem 57(6): 856-863.
- Yamanishi M, Koba S, Jo T, Kotera T, Imashuku S (2018) Thrombotic Microangiopathy-Like Hemolysis in Vitamin B12 Deficiency-Related Macrocytic Anemia. Clin Lab 64(4): 639-643.
- Tran PN, Tran MH (2018) Cobalamin deficiency presenting with thrombotic microangiopathy (TMA) features: A systematic review. Transfus Apher Sci 57(1): 102-106.
- Toh BH, Whittingham S, Alderuccio F (2006) Gastritis and pernicious anemia. Autoimmune Dis 39: 527-546.
- Lahner E, Annibale B Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol 15(41): 5121-5128.
- Chan CQ, Low LL, Lee KH (2016) Oral Vitamin B12 Replacement for the Treatment of Pernicious Anemia. Front Med (Lausanne) 3: 38.
- Andrès E, Zulfiqar AA, Vogel T (2019) State of the Art Review: Oral and nasal vitamin B12 therapy in the elderly. QJM 22 pii: hcz046.
- Bizzaro N, Antico A (2014) Treatment of pernicious anemia: Which is the best option? Autoimmun Rev 13(7): 779.
- Gallego-Narbón A, Zapatera B, Álvarez I, Vaquero MP (2018) Methylmalonic Acid Levels and their Relation with Cobalamin Supplementation in Spanish Vegetarians. Plant Foods Hum Nutr 73(3): 166-171.
- Wang H, Li L, Qin LL, Song Y, Vidal-Alaball J, et al. (2018) Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 Cochrane Database Syst Rev 3: CD004655.
- Senol MG, Sonmez G, Ozdag F, Saracoglu M (2008) Reversible myelopathy with vitamin B12 deficiency. Singap Med J 49(11): e330-e332.
- Okada S, Kuwako T, Nakajo H, Ishihara M, Uchiyama F, et al. (2006) Two cases of subacute combined degeneration: Magnetic resonance findings. J Nippon Med Sch 73(6): 328-331.






























