Electromagnetic Therapy for Neurological and Neurodegenerative Diseases: I Peripheral Brain Stimulations
Alain L Fymat*
International Institute of Medicine & Science, USA
Submission: October 25, 2019;Published: December 04, 2019
*Corresponding author: Alain L Fymat, International Institute of Medicine & Science, California, USA
How to cite this article: Alain L Fymat. Electromagnetic Therapy for Neurological and Neurodegenerative Diseases: I Peripheral Brain Stimulations . Open Access J Neurol Neurosurg. 2019; 12(2): 555833. DOI: 10.19080/OAJNN.2019.12.555833.
Abstract
Electrical and magnetic field currents can produce functionally reversible stimulations. This property has been used for diagnostic and therapeutic neurosurgical procedures. The various known electrical and magnetic procedures are separately studied and compared relative to their types, effects, indications, contraindications, adverse events, and benefits. As of 2015, transcranial direct current stimulation (a Class III device) has not been approved for any use in the U.S. However, it is an approved treatment for major depressive disorder in the European Union, Australia, and Mexico. It induces changes in neurohormones, and neurotransmitters implicated in psychiatric disorders. Electroconvulsive (or electroshock) therapy is a psychiatric treatment that induces seizures in patients to provide relief from mental disorders. It is safe and effective for treatment-resistant major depressive disorder and catatonia, and prolonged or severe mania. However, its use remains controversial. Functional electrical stimulation, originally used to develop neuroprostheses, is a short-term intervention to help the central nervous system re-learn how to execute impaired functions, obviating the need for permanent prostheses. Common applications of the vagus nerve and transcutaneous vagus nerve stimulation are in epilepsy, migraines and fibromyalgia. Similar to magnetic resonance imaging, transcranial magnetic stimulation is applied for diagnostic and therapeutic potential in a wide variety of disease states in neurology and mental health, in the measurement of the activity and function of specific brain circuits in humans, in the measurement of the connection between the primary cortex and to evaluate damage related to past or progressive neurologic insult. It has potential applications to several other neurologic and psychiatric conditions, and to mapping the functional connectivity between the cerebellum and other areas of the brain. Under current investigation, magnetic seizure therapy has a number of purported applications including treatment-resistant depression, schizophrenia, obsessive-compulsive disorder, and psychiatry. On the other hand, pulsed electromagnetic field therapy has applications in delayed and non-union fractures, depression, and knee osteoarthritis. A synthesis of all of the above procedures is provided in terms of their indications, contraindications, benefits, and adverse effects [1-10].
Keywords: Cranial electrotherapy stimulation; Electroconvulsive (or electroshock) therapy; Functional electrical stimulation; Low-field magnetic stimulation; Magnetic seizure therapy; Neuromuscular electrical stimulation; Psychosurgery; Transcranial electrical stimulation; Transcranial magnetic stimulation; Transcranial random noise stimulation; Transcranial vagus nerve stimulation
Abbrevations: A: Ampere; AC: Alternating Current; AD: Alzheimer’s Disease; ADL: Activities of Daily Living; ALS: Amyotrophic Lateral Sclerosis (aka Lou Gehring disease); APA: American Psychiatric Association; C: Coulomb; CE: Communaute Europeenne (European Community); CES: Cranial Electrotherapy Stimulation; CHF: Chronic Heart Failure; CT: Computerized Tomography; DC: Direct Current; ECT: Electroconvulsive Therapy; EEG (or EKG): Electroencephalography; EM: Electromagnetic (field); EST: Electroshock Therapy; FDA: (U.S.) Food & Drug Administration; FES: Functional Electrical Stimulation; Gpi: Globus Pallidus Pars Internus; FMRI: Functional MRI; HRSD: Hamilton Rating Scale for Depression; IPG: Implantable Pulse Generator; LFMS: Low-Field Magnetic Stimulation; MCI: Mild Cognitive Impairment; MDD: Major Depressive Disorder; MOI: Monoamine Oxidase Inhibitors; MRI: Magnetic Resonance Imaging; MS: Multiple Sclerosis; MST: Magnetic Seizure Therapy; NDD: Neurodegenerative Diseases; NICE: (U.K.) National Institute for Health and Clinical Excellence; NIH: (U.S.) National Institutes of Health; NMES: Neuromuscular Electrical Stimulation; OCD: Obsessive-Compulsive Disorder; PD: Parkinson’s Disease; PNS: Peripheral Nervous System; PTSD: Post-Traumatic Stress Disorder; QOL: Quality Of Life; RCT: Randomized Clinical Trials; RMP: Resting Membrane Potential; rTMS: Repetitive Transcranial Magnetic Stimulation; rTVNS: Repetitive Transcranial Vagus Nerve Stimulation; SNC: Substantia Nigra Pars Compacta; STN: Sub-Thalamic Nuclei; TES: Transcranial Electrical Stimulation; TACS: Transcranial Alternating Current Stimulation; TDCS: Transcranial Direct Current Stimulation; TMS: Transcranial Magnetic Stimulation; TRNS: Transcranial Random Noise Stimulation; TVNS: Transcutaneous Vagus Nerve Stimulation; V+/-: (Positive, Negative) Voltage; VIM: Ventral Intermediate Nucleus of the Thalamus; VNS: Vagus Nerve Stimulation
Drugs Cited
Amantadine; Ambien; Camphor; Levodopa; Metrazol (Cardiazol); Prozac
Diseases/Disorders Listed
Alzheimer’s disease; Asystole; Amyotrophic lateral sclerosis (Lou Gehring disease); Brain arteriovenous malformations; Catatonia; Cerebral palsy; Cognitive impairment; Developmental delay; Drop foot; Epilepsy; Glioblastoma; Heart arrhythmia; Hemiplegia; Huntington’s chorea; Hydrocephalus; Mild cognitive impairment; Major depressive disorder; Mania; Multiple sclerosis; Neurodegenerative disease; Osteoarthritis; Parkinson’s disease; Persistent vegetative state; Schizophrenia; Spinal cord injury; Stroke; Stroke-related disability, Tinnitus; Traumatic brain injury
Introduction
Electrical and magnetic field currents can produce functionally reversible stimulations. This property has been used for diagnostic and therapeutic neurosurgical procedures. The history of these developments is first briefly recounted beginning since the dawn of the therapeutic applications in the mid-16th century. For convenience, Electromagnetic (EM) stimulations have been separated into electrical and magnetic stimulations. The former include transcranial electrical stimulation (with either direct or alternating current), electroconvulsive (or electroshock) therapy that remains controversial, functional (or neuromuscular) electrical stimulation for paralysis due to injury, and vagus nerve stimulation and its transcutaneous variation. The latter include transcranial magnetic stimulation, magnetic seizure therapy, and pulsed electromagnetic field therapy (or low-field magnetic stimulation, or tumor treating fields). These several modalities are compared in their types, effects, indications, contraindications, adverse events, and benefits [11-45].
A Brief History of Electromagnetic Brain Stimulations
The history of electromagnetic brain stimulations is over three centuries old. Its major steps and hallmarks are summarized below:
Mid-16th century: Agents to induce seizures are used to treat psychiatric conditions.
1744: Dawn of electricity’s therapeutic use (as documented in the first issue of Electricity and Medicine).
1755: Treatment and cure of hysterical blindness is documented. (Benjamin Franklin wrote that an electrostatic machine cured a woman of hysterical fits.)
Mid-18th century: Luigi Galvani (1737-1798) discovers that nerves and muscles are electrically excitable, laying the foundations for the field of electrophysiology
1785: Therapeutic use of seizure induction is documented in the London Medical & Surgical Journal.
Late-18th century: Giovanni Aldini (nephew of Galvani), Alessandro Volta, and others studied low- i n t e n s i t y , galvanic (electrical) currents in humans and animals. (Aldini had experimented with galvanic head current upon himself as early as 1794).
1800s: Michael Faraday discovers that an electrical current had a corresponding magnetic field and that changing one could induce its counterpart.
1801-4: Giovanni Aldini reported the successful treatment of patients suffering from mental disorders and melancholia using direct low-intensity currents.
Early to mid-19th century: Electrical brain stimulation is pioneered by Luigi Rolando (1773-1831) and Pierre Flourens (1794-1867) for the study of the brain localization of function.
1841: Early claims that electricity might aid bone healing.
Mid-19th century: Eduard Hitzig (1838-1907), Gustav Fritsch (1838-1927), David Ferrier (1842-1928) and Friedrich Goltz (1834-1902) stimulate the surface of the cerebral cortex to investigate the motor complex in animals.
Mid-19th century: Electroshock therapy-type treatments were notable in British asylums
Late 19th and early 20th century: Robert Bartholow (1831- 1904) and Fedor Krause (1857-1937) e l e c t r i c a l l y stimulate the human brain cortex.
Early 20th-century: Work continues for the direct stimulation of the human brain with electricity and Victor Horsley (1857-1916) improves the invention of the stereotactic method.
1930: Cerletti and Bini’s electroconvulsive therapy is widely used for the treatment of mental illness and, ultimately, overused as it began to be seen as a panacea, leading to a backlash in the 1970s.
Early-to mid-20th century: Walter Rudolf Hess (1881-1973), Jose Delgado (1915-2011), and others develop chronic electrode implants to be inserted deep into the brain of cats and monkeys.
1934: Convulsive therapy is introduced by the Hungarian neuropsychiatrist Ladislas J. Medunawho, b e l i e v i n g mistakenly that schizophrenia and epilepsy were antagonistic disorders. He induced seizures first with camphor and then metrazol (cardiazol).
1937: The first international meeting on schizophrenia and convulsive therapy is held in Switzerland by the Swiss psychiatrist Max Müller. The proceedings were published in the American Journal of Psychiatry and, within three years, cardiazol convulsive therapy is being used worldwide.
1938: Italian Professor of neuropsychiatry Ugo Cerletti (who had been using electric shocks to produce seizures in animalexperiments) and his colleague Lucio Bini use electricity as a substitute for metrazol in convulsive therapy and experiment for the first time on a person.
1938: First electroconvulsive (electroshock) therapy is conducted.
1940: Electroconvulsive therapy is introduced in England, the U.S., Germany, and Austria.
1940s: Electroconvulsive (electroshock) therapy becomes popular in the U.S.
1940s-1950s: The use of electroconvulsive therapy becomes widespread.
Mid-20th century: Utilizing electrode implantation by the electrostatic method, Wilder Penfield (1891- 1976), Robert Galbraith Heath, Jose Delgado, and their colleagues use extensively electrical stimulation of the brain cortex in awake patients to investigate the motor and sensory homunculus (this is the representation of the body in the brain cortex according to the distribution of motor and sensory territories.) Note that to a few exceptions, emotional reaction in humans could not be elicited by either observing spontaneous epilepsy or electrically stimulating the surface of the cerebral cortex.
The 1950s-1960s: Marked decline in the use of electroconvulsive therapy due to the steady growth in the use of antidepressants with negative depictions of electroconvulsive therapy in the mass media.
The 1960s: DJ Albert proves that stimulation could affect brain function by changing cortical excitability. He also discovers that positive and negative stimulation had different effects on the cortical excitability.
Around 1967: Functional electrical stimulation was first used to treat foot drop by stimulating the peroneal nerve during gait. (A switch located in the heel-end of a user’s shoe would activate a stimulator worn by the user.)
The 1970s: Publication of the first American Psychiatric Association’s Task Force Report on Electroconvulsive Therapy” (to be followed by further reports in 1990 and 2001). The report endorses the use of electroconvulsive therapy in the treatment of depression. Also, Bassett et al. introduce a new technique employing a very specific biphasic low-frequency signal to be applied for non-union/delayed fractures
1976: Blatchley demonstrates the effectiveness of his constant current, brief pulse device electroconvulsive therapy, which led to a reduction in cognitive side effects.
1977: Carnstam et al. show that functional electrical stimulation can treat foot drop in people with multiple sclerosis by generating strength increases through using peroneal stimulation.
1980: Merton and Morton successfully use transcranial electrical stimulation to stimulate the motor cortex.
The 1980s: Prozac is introduced to treat depression, anxiety, and insomnia.
1985: Anthony T. Barker explores the use of magnetic fields to alter electrical signaling within the brain, developing the first stable transcranial magnetic stimulation device. Also, the (U.S.) National Institute of Mental Health of the National Institutes of Health convenes a consensus development conference on electroconvulsive therapy and concludes that while electroconvulsive therapy was the most controversial treatment in psychiatry and had significant side-effects, [it] was effective for a narrow range of severe psychiatric disorders.
The 1990s: Ambien is introduced to treat depression, anxiety, and insomnia.
1994: Kralj, Graupe develop a functional electrical stimulation system (the Parastep) to enable spinal cord injury people to stand and walk.
2001: The American Psychiatric Association releases its latest Task Force Report that emphasized the importance of informed consent and the expanded role that the procedure has in modern medicine.
2004: The (U.S.) Food & Drug Administration (FDA) approves a pulsed electromagnetic field system as an adjunct to cervical fusion surgery in patients at high risk for non-fusion.
Around 2005: The combination of pharmaceutical brands becoming generic and Internet advertising cause cranial electrotherapy stimulation devices to gain popularity [46-80].
2005: The FDA approves a vagus nerve stimulation device for treatment-resistant major depressive disorder.
2008: The FDA authorizes the use of repetitive transcranial magnetic stimulation for the treatment of depression that has not improved with other measures. Several deep transcranial magnetic stimulation devices have received FDA clearance for use in adults with treatment-resistant major depressive disorders
2009: Nexstim obtains FDA clearance for the assessment of the primary motor cortex for neurological pre-procedural planning.
2011: Nexstim obtains FDA clearance for neurosurgical planning. Also, cranial electrotherapy s t i m u l a t i o n devices receive media attention from the Wall Street Journal. Further, the FDA’s Neurological Devices Advisory Panel recommends that FDA maintain electroconvulsive therapy devices in the Class III category for high-risk devices, except for patients suffering from catatonia, major depressive disorder, and bipolar disorder.
2013: Dwyer uses electrical stimulation in the lumbosacral region. Also, the FDA approves the use of s i n g l e - p u l s e transcranial magnetic stimulation for the treatment of migraines.
2014: The FDA determines that there is sufficient information to establish general and special controls to provide a reasonable assurance of safety and effectiveness for cranial electrotherapy stimulation devices.
2015: The FDA approves a vagus nerve stimulation device for treatment-resistant major depressive disorder.
2017-18: The FDA approves gamma Core, a handheld noninvasive vagus nerve stimulator made by Electro Core LLC, for episodic cluster headaches and migraine pain.
2018: The FDA authorizes the use of transcranial magnetic stimulation in the treatment of obsessive- c o m p u l s i v e disorder.
2019: The FDA clears the wearable Recovery Rx PEMF Device for adjunctive treatment of postoperative pain
Distilled from this past history, I review and analyze below the several types of electromagnetic stimulations
Electrical Stimulations and their Therapeutic Applications
Transcranial direct current stimulation
Transcranial direct current stimulation TDCS is a form of neuromodulation that uses constant Direct Current (DC) of low intensity delivered through electrodes positioned on the head. When the electrodes are placed in a region of interest, the current induces intracerebral current flow. This current flow then either increases or decreases the neuronal excitability in the specific area being stimulated depending on the type of stimulation used. There are three different types of stimulation: anodal, cathodal, and sham. The anodal stimulation (positive voltage: V+) is a positive stimulation that increases the neuronal excitability of the area being stimulated; cathodal (V-) stimulation is the opposite, and sham stimulation is a control. This change of neuronal excitability leads to alteration of brain function, which can be used in various therapies as well as to provide more information about the functioning of the human brain [81-100].
TDCS was originally developed to help patients with brain injuries or presenting psychiatric conditions like Major Depressive Disorder (MDD). It was considered a safe and effective treatment for depression. It was contraindicated for people susceptible to seizures such as epileptic patients. Short-term adverse effects and contraindications of the procedure include skin irritation, a phosphene at the start of stimulation (this is a brief flash of light if the electrode is placed near the eye), nausea (when the electrodes are placed above the mastoid for stimulation of the vestibular system), headache, dizziness, and itching. Long-term adverse effects are unknown. In cathodal stimulation, animal(rat) experiments designed to determine the current density at which overt brain damage occurs showed that a current density of 142.9 [A/m2] (Ampere/square meter) delivering a charge density of 52,400 [C/m2] (Coulomb/square meter), or higher, caused a brain lesion. This dose is over two orders of magnitude higher than the dose in protocols for human patients. Interestingly, TDCS can achieve cortical changes even after the stimulation has ended depending on the intensity (strength) and the duration of the stimulation. The effects increase with the increase on either/ or both of these two factors. Stimulation changes brain function either by causing the neuron’s Resting Membrane Potential (RMP) to depolarize or hyperpolarize. In positive (anodal) stimulation, the current causes a depolarization of the RMP, increasing neuronal excitability and allowing for more spontaneous cell firing. By contrast, in negative (cathodal) stimulation, the current causes a hyperpolarization of the RMP, decreasing neuron excitability. Using multiple smaller sized gel electrodes rather than two large ones allows greater and longer-lasting motor cortex excitability [101-120].

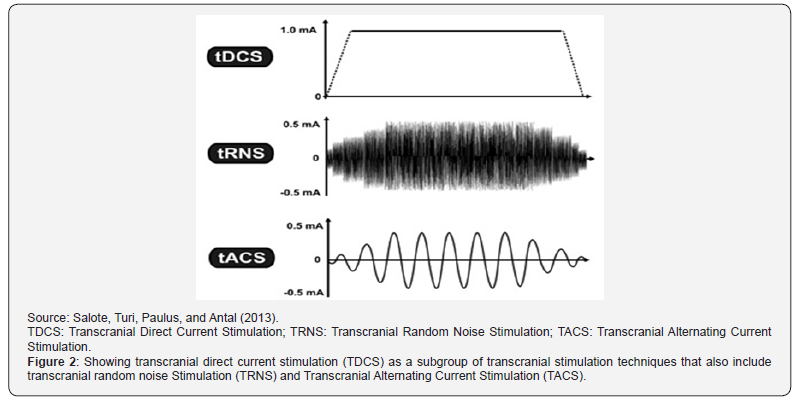
Cathodal stimulation can treat psychiatric disorders caused by the hyperactivity of an area of the brain. Sham stimulation is used as a control in the experiments. It emits a brief current but then remains off for the remainder of the stimulation time. With sham stimulation, the person receiving the TDCS does not know that he/she is not receiving prolonged stimulation. By comparing the results in subjects exposed to sham stimulation with the results of subjects exposed to anodal (positive) or cathodal (negative) stimulation, researchers can determine how much of an effect is caused by the current stimulation, rather than by the placebo effect. Figure 1 illustrates the administration of TDCS. The anodal and cathodal electrodes are respectively put on the FS and supraorbital regions. Figure 2, where the vertical axisrepresents the current intensity in [mA] (milli Amperes) and the horizontal axis illustrates the time-course, shows that TDCS is but a specific subgroup of non-invasive brain stimulation techniques that includes Transcranial Random Noise Stimulation (TRNS) and Transcranial Alternating Current Stimulation (TACS), as further discussed in the next section[121-140].
Regarding the applicability of the procedure, results of randomized clinical trials (RCT) indicate:
a. Amelioration of memory deficits in Parkinson’s (PD) and Alzheimer’s Disease (AD), schizophrenia, neuropathic pain after spinal cord injury, non-neuropathic pain: good evidence.
b. Cognitive enhancement in healthy people: no statistically conclusive evidence.
c. Improvement in Activities of Daily Living (ADL) in PD: evidence of very low to moderate quality
d. Improvements of fibromyalgia, depression, and craving: level B evidence (probable efficacy).
e. Neurophysiological outcome: little-to-no statistically reliable impact.
f. Alleviation of symptoms of Major Depressive Disorders (MDD): inconclusive evidence.
g. Schizophrenia symptoms improvement: variable evidence.
h. Pain alleviation: low-quality research, cannot be used as a basis to recommend the use of TDCS to treat pain.
i. Chronic pain following spinal cord injury: high-quality research showing TDCS is ineffective.
j. Stroke: TDCS is not effective for improving upper limb function after stroke
k. Post-stroke aphasia no improvement from combining TDCS with conventional treatment.
l. Vision deficits following stroke TDCS may be effective.
m. Various psychiatric disorders (depression, reverse cognitive deficits, improvement of focus and concentration): inconclusive evidence from the studies conducted.
Neuroscience research aims to
a. link specific brain regions to specific cognitive tasks or psychological phenomena and
b. to study the functional connectivity of the cerebellum to other areas of the brain. The cerebellum has been a focus of research, due to its high concentration of neurons, its location immediately below the skull, and its multiple reciprocal anatomical connections to motor and associative parts of the brain. Most such studies focus on the impact of cerebellar TDCS on motor, cognitive, and affective functions in healthy and patient populations.
As of 2015, TDCS has not been approved for any use in the U.S. However, it is an approved treatment for MDD in the EU, Australia, and Mexico. Variants related to TDCS include transcranial alternating current stimulation (TACS) and Transcranial Random Noise Stimulation (TRNS), a group of technologies commonly referred to as Transcranial Electrical Stimulations (TES) [141- 160].
Transcranial alternating current electrical stimulation
Also known as cranial-electrostimulation and transcranial electrotherapy, Transcranial Alternating Current Stimulation (TACS) is a form of neurostimulation that delivers a small, pulsed, alternating current via electrodes in the head. It was initially studied for insomnia and called electro sleep therapy. It is also used for treating headaches, neuromusculoskeletal pain, fibromyalgia, degenerative joint pain, smoking cessation. and opiate withdrawal. There is modest evidence for benefits in anxiety and depression (although it is not sufficient for use in acute depression) but little evidence of safety and effectiveness for many of the other conditions. Despite its long history, the underlying principles and mechanisms of TACS are still unclear. In practice, electrodes are placed on the ear lobes, maxilla-occipital junction, and mastoid processes or temples to stimulate the thalamic area and the cortical and subcortical areas. TACS treatments induce changes in neurohormones and neurotransmitters implicated in psychiatric disorders: substantial increases in beta-endorphins, adrenocorticotrophic hormone, and serotonin; moderate increases in melatonin and norepinephrine; modest or unquantified increases in cholinesterase, gamma-aminobutyric acid, and dehydroepiandrosterone; and moderate reductions in cortisol. In the U.S., the TACS technology is classified by the Food & Drug Administration (FDA) as a Class III medical device that must be dispensed by, or on the order of, a healthcare practitioner (physician, psychiatrist, nurse practitioner, psychologist, physician assistant, or occupational therapist) having an appropriate electrotherapy license. There are eleven TACS devices cleared formarketing in the US.
Electroconvulsive (or electroshock) therapy
Electroconvulsive Therapy (ECT), formerly known as Electroshock Therapy (EST), is a psychiatric treatment in which seizures are electrically induced in patients to provide relief from mental disorders. Short-term, it works via an anticonvulsant effect mostly in the frontal lobes and longer-term via neurotrophic effects primarily in the medial temporal lobe. Administered under anesthesia with a muscle relaxant, the procedure is safe and effective for treatment-resistant major depressive disorder, treatment-resistant catatonia, prolonged or severe mania, and in treatments where there is a need for rapid, definitive response because of the severity of a psychiatric or medical condition, e.g., when illness is characterized by stupor, marked psychomotor retardation, depressive delusions, hallucinations or life-threatening physical exhaustion associated with mania). Treatment involves multiple administrations, typically given two or three times per week until the patient is no longer suffering symptoms. In its application, ECT differs in three different ways accompanied by significantly different symptom remission and adverse side effects.
a. Electrode placement (unilateral in which the current is passed through one brain hemisphere; more efficacious bilateral for both hemispheres but greater risk of memory loss),
b. Frequency of treatments, and
c. Electrical waveform of the stimulus.
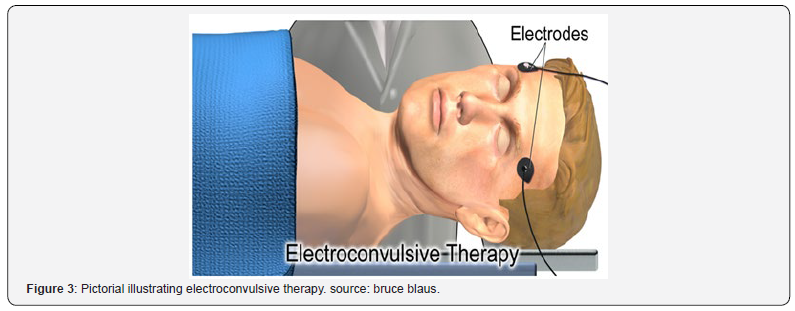
Drug therapy is usually continued after treatment and some patients receive maintenance ECT. The schematic of Figure 3 illustrates the electroconvulsive therapy. ECT is effective in about 50% of people with treatment-resistant major depressive disorder (unipolar or bipolar), half of whom relapse within 12 months. The most common adverse effects are confusion and transient memory loss, and muscle soreness invariably due to the administration of muscle relaxants. General physical risks are similar to those of brief general anesthesia rather than caused by muscle activity. The death rate during ECT is around 4 per 100,000 procedures. Precautions should, however, be taken for patients who have unstable or severe cardiovascular conditions or aneurysms, have suffered a recent stroke, have increased intracranial pressure (for instance, due to a solid brain tumor), are afflicted by severe pulmonary conditions, or are generally at high-risk for receiving anesthesia. In adolescents, ECT is highlyefficient for several psychiatric disorders, with few and relatively benign adverse effects.
Common applications include more specifically
Major depressive disorder: ECT is generally used only when other treatments have failed or in emergencies such as imminent suicide. It has also been used in selected cases of depression occurring in the setting of multiple sclerosis (MS), Parkinson’s Disease (PD), Huntington’s chorea, developmental delay, brain arteriovenous malformations, and hydrocephalus
Unipolar and bipolar depression patients respond equally well to ECT whereas they respond differently to other medical treatments. However, there is little agreement on the most appropriate follow-up. Thus, without antidepressants, 74% of people relapse; with antidepressants, about 50% relapse within 12 months, and about 37% relapse within the first 6 months. In terms of efficacy, ECT is superior to stimulation, placebo, antidepressants, tricyclics, and Monoamine Oxidase Inhibitors (MOI). Compared with transcranial magnetic stimulation (TMS), ECT relieves depression about twice as well, reducing the score on the Hamilton Rating Scale for Depression (HRSD) by about 15 points, while TMS reduced it by only 9 points.
Catatonia
Generally, ECT is a first-line treatment for severe or lifethreatening catatonia but second line for unresponsive catatonia. Notwithstanding the absence of randomized clinical trials for efficacy, ECT is generally acknowledged to be excellent. Unfortunately, there is little published evidence in the case of added autism spectrum disorders.
Mania
While ECT is used to treat people, who have severe or prolonged mania, the (UK) National Institute for Healthcare and Clinical Excellence (NICE) recommends it only in life-threatening situations, when other treatments have failed, and as a secondline treatment for bipolar mania.
Schizophrenia
While widely used worldwide in the treatment of schizophrenia, in North America and Western Europe, ECT is invariably used only in treatment-resistant illness when there is little response to antipsychotics alone. ECT is useful in severe exacerbations of catatonic schizophrenia, whether excited or stuporous.
Cognitive impairment
After ECT, the following may sometimes be noticed: cognitive impairment, incomplete recovery from retrograde amnesia (for events occurring before the treatment) and anterograde amnesia (for events occurring after the treatment), and persistent or permanent memory loss and confusion. The memory losses are minimized with the use of brief pulse currents.
Effects on brain structure
Notwithstanding controversy on the subject, the American Psychiatric Association (APA) has concluded that there is no evidence that ECT causes structural brain damage.
Effects in pregnancy
If steps are taken to decrease potential risks (pelvic examination, discontinuation of non-essential anticholinergic medication, uterine tocodynamometry, intravenous hydration, and administration of a nonparticulate antacid), ECT is generally accepted to be relatively safe during all trimesters of pregnancy, particularly when compared to pharmacological treatments. In many instances of active mood disorder, the risks of untreated symptoms may outweigh the risks of ECT.
Effects on the heart
ECT can cause a lack of blood flow and oxygen to the heart, heart arrhythmia, and “persistent asystole” (of two or more seconds in just under 50% of patients) but, in all such cases, the heartbeat returned without any intervention. Although heart failure is exceedingly rare as a complication, it can occur, and deaths during ECT are usually due to cardiovascular complications. within the psychiatric and wider medical community, surveys of public opinion, testimonies of former patients, legal restrictions on the use of ECT, disputes as to its efficacy, ethics, and adverse effects indicate that the use of ECT remains controversial.
Functional (or neuromuscular) electrical stimulation
Functional Electrical Stimulation (FES) or Neuromuscular Electrical Stimulation (NMES) uses low-energy electrical pulses to artificially generate body movements in individuals paralyzed due to injury to their Central Nervous System (CNS). It generates muscle contraction in otherwise paralyzed limbs, producing functions such as grasping, walking, bladder voiding, and standing. The technology was originally used to develop neuroprostheses as permanent substitutes for impaired functions in individuals with spinal cord injury (SCI), head injury, stroke, other neurological disorders, and therapies to restore voluntary function. It is a short-term intervention to help the person’s CNS re-learn how to execute impaired functions, obviating the need for permanent prostheses.
FES stimulates neurons, nerves, and denervated muscles whose peripheral nerves have been severed or damaged but mostly the junctions between the nerves and muscles. The electrical charge can stimulate both motor nerves (efferent nerves descending from the CNS to muscles) and sensory nerves (afferent nerves ascending from sensory organs to the CNS). The electrical charge can stimulate both motor and sensory nerves. When a nerve is stimulated, a localized depolarization of the cell wall occurs resulting in an action potential that propagates toward both ends of the axon. Typically, one “wave” of action potential will propagate along the axon towards the muscle (orthodromic propagation) and, concurrently, the other waveof action potential will propagate towards the cell body in the CNS (antidromic propagation). Typically, FES is concerned with orthodromic stimulation and uses it to generate coordinated muscle contractions.
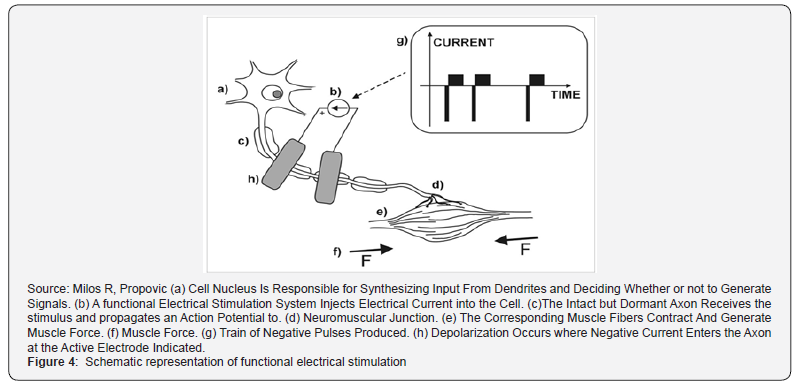
Nerves can be stimulated using either surface (transcutaneous) or subcutaneous (percutaneous or implanted) electrodes. The surface electrodes are placed on the skin surface above the nerve or muscle that needs to be activated. They are noninvasive, easy to apply, and generally inexpensive. A major limitation of the transcutaneous electrical stimulation is that some nerves are too profound to be stimulated using surface electrodes. This limitation can be partly addressed by using arrays of electrodes, which employ several electrical contacts to increase selectivity. One of the drawbacks of percutaneous electrodes is that they are prone to infection, requiring special care. Figure 4 illustrates motor neuron stimulation in FES. Compared to surface stimulation electrodes, implanted and percutaneous electrodes potentially have higher stimulation selectivity. The drawback of the implanted electrodes is that they require an invasive surgical procedure to install and there exists a possibility of infection following implantation.
Common applications include
Cerebral palsy
FES has led to significant orthotic and training effects in children with unilateral spastic cerebral palsy with improvements in muscle mass and strength, gastrocnemius spasticity, passive range of motion, upper extremity function, walking speed, positioning of the foot, ankle kinematics, community mobility, and balance skills.
Drop foot
Drop foot is a common symptom in hemiplegia. It is characterized by a lack of dorsiflexion during the swing phase of gait, resulting in short, shuffling strides. FES can effectivelycompensate for this condition during the swing phase of the gait. Several drop foot stimulators use surface and implanted FES technologies. They have been used successfully with various patient populations, such as spinal cord injury, stroke, and multiple sclerosis. The (UK) NICE has issued the following guideline on the treatment of drop foot of central neurological origin. current evidence on the safety and efficacy (in terms of improving gait) of Functional Electrical Stimulation (FES) for drop foot of central neurological origin appears adequate to support the use of this procedure provided that normal arrangements are in place for clinical governance, consent and audit.
Multiple sclerosis
FES is useful for treating drop foot in people with multiple sclerosis generating strength increases through peroneal stimulation to show an orthotic effect for walking speed.
Spinal cord injury
Spinal cord injuries (SCI) interfere with electrical signals between the brain and the muscles, resulting in paralysis below the level of injury. Restoration of limb function, regulation of organ function, pain treatment, pressure, and sore prevention are the main applications of FES. Examples include the use of neuroprostheses to allow paraplegics to walk and stand, restore hand grasp function in quadriplegics, restore bowel and bladder function, increase muscle mass and fiber diameter, improve the ultrastructural organization of contractile material, increase force output during electrical stimulation, and perform FES-assisted stand-up exercises. Prime examples are the Kralj. FES system (the Parastep) that enables spinal cord injury people to stand and walkwith some exceeding one mile per walk and the Complex Motion neuroprosthesis for exercise and as an alternative to wheelchair mobility.
Stroke and upper limb recovery
In the acute stage of stroke recovery, cyclic electrical stimulation increases the isometric strength of wrist extensors. Patients who will elicit benefits must be highly motivated to follow through with treatment. Hemiplegic patients following a stroke commonly experience shoulder pain and subluxation both of which will interfere with the rehabilitation process. Over time (24 months or more), FES is effective for the management of pain, reduction of shoulder subluxation, and acceleration of the degree and rate of motor recovery. Hemiparetic stroke patients who are impacted by denervation, muscular atrophy, and spasticity typically experience an abnormal gait pattern due to muscular weakness and are unable to voluntarily contract certain ankle and hip muscles at the appropriate walking phase. FES can help in this situation.
Vagus nerve stimulation and transcutaneous vagus nerve stimulation
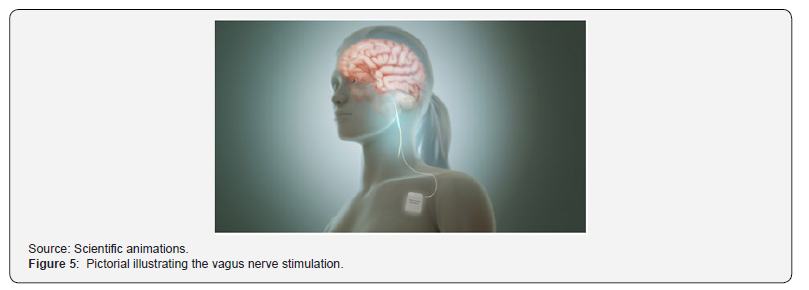
The vagus is the tenth cranial nerve. It arises from the medulla and carries both afferent and efferent fibers. The afferent vagal fibers connect to the nucleus of the solitary tract that, in turn, projects connections to other locations in the CNS. Vagus Nerve Stimulation (VNS) delivers electrical pulses to the vagus nerve. Proposed mechanisms include an anti-inflammatory effect as well as changes in monoamines. The device, a generator the size of a matchbox, is implanted under the skin below the collarbone. Lead wires from the generator are tunneled-up to the patient’s neck and wrapped around the left vagus nerve at the carotid sheath, where it delivers electrical impulses. The left vagus nerve is stimulated rather than the right because the right plays a role in cardiac function such that stimulating it and could have negative cardiac effects. Figure 5 illustrates the medical stimulation of the vagus nerve.
Common applications include
Epilepsy
It is an add-on treatment to treat drug-resistant epilepsy (specifically focal epilepsy) and treatment-refractory Major Depressive Disorder (MDD). In epilepsy, adverse events include moderate-to-severe increase in seizures, cardiac arrest, bradycardia, voice-alteration and hoarseness, trouble talking, coughing, shortness of breath, pain, a tingling sensation, nausea, headache, difficulty swallowing, and sleepiness. The efficacy and side effects of the procedure in MDD are less clear.
Major depressive disorder
VNS is an add-on for treatment-refractory major depressive disorder
Other applications
functions and brain regions, clinical research has been done to determine its usefulness in treating other illnesses, including various anxiety disorders, obesity, alcohol addiction, chronic heart failure, prevention of arrhythmias that can cause sudden cardiac death, autoimmune disorders, several chronic pain conditions, and neurodevelopmental disorders including Landau-Kleffner syndrome, Rett syndrome, autism spectrum disorders, stroke, and depression.
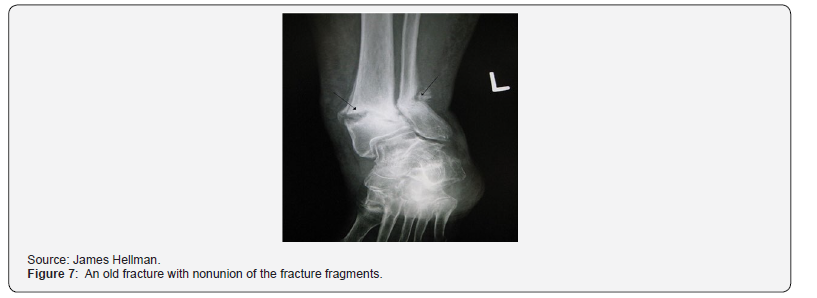
As of 2015, Transcutaneous Vagus Nerve Stimulation (TVNS) devices were being developed for electrical transmission through the skin for the treatment of migraines and fibromyalgia based on the rationale that there is vagus nerve distribution on the ear’s surface. Electrical impulses are targeted at either the auricle of the auricular branch of the vagus nerve or the cervical vagus nerve. Table 1 provides a comparison of transcranial electrical stimulation techniques in terms of their indications and contraindications, and advantages and adverse effects.
Magnetic Stimulations and their Therapeutic Applications
Transcranial magnetic stimulation
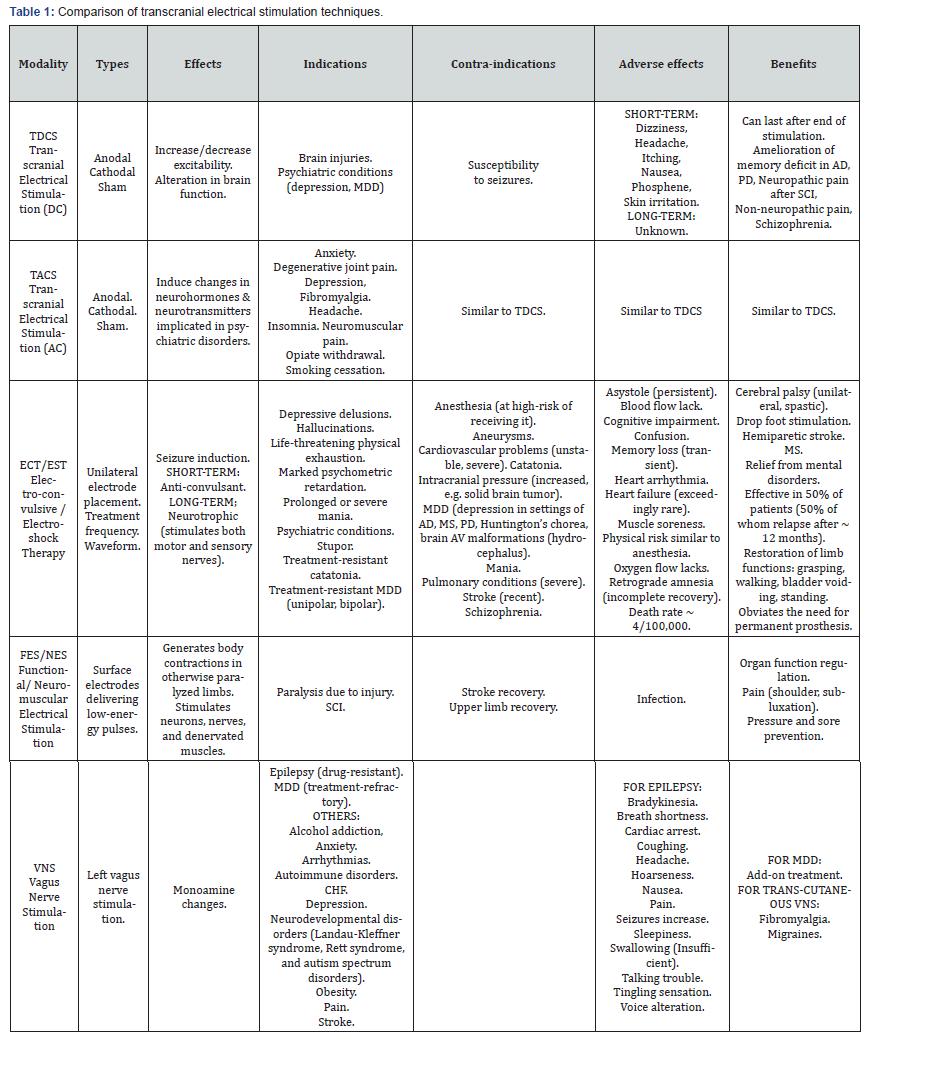
Transcranial Magnetic Stimulation (TMS) is a noninvasive means of imparting electrical energy across the head’s insulating tissues and into the brain. An electrical current is passed through a coil of wire placed above the region of interest on the scalp. Through electromagnetic induction, the current generates rapidly changing magnetic fields that induce small electrical currents in specific areas of the brain. Oriented perpendicular to the plane of the coil, the magnetic field passes virtually unimpeded through the scalp and skull. In the brain, it produces currents in the induced electrical field lying parallel to the plane of the coil. These currents can excite neural processes lying in the plane of the induced field in a manner roughly analogous to direct cortical stimulation with electrodes (Figure 6 &7).
Two TMS types (single pulse, repetitive pulse) are used in research therapy. Effects lasting longer than the stimulation period are only observed in repetitive TMS (rTMS). Like for TDCS, an increase or decrease in neuronal activity can be achieved, but the induction method is very different. In TDCS, the two different directions of current cause the different effects. By contrast, in rTMS, increased/decreased neuronal activity is induced by using a higher/lower frequency.
An electric pulse generator, or stimulator, is connected to a magnetic coil that is connected to the scalp. It leads to a cascade of effects: generation of a changing electric current within the coil, induction of a magnetic field, production of a second inductance of inverted electrical charge within the brain that activates nearby nerve cells, and further production of a localized electrical current which can then either depolarize or hyperpolarize neurons at that site. Effects vary based on the frequency, intensity, and length of the magnetic pulse. While similar to Magnetic Resonance Imaging (MRI), the pulse here generally penetrates no deeper than 5 centimeters unless a modified coil technique is used to reach deeper stimulation. Deep TMS can reach up to 6 cm into the brain to stimulate deeper layers of the motor cortex to control leg motion. TMS can be a physiological probe of cortical function for clinical and basic neurophysiology.
Common applications include
Diagnostic and therapeutic potential in a wide variety of disease states in neurology and mental health. Adverse effects are rare and more pronounced for rTMS than for single-pulse TMS and include fainting (uncommon) and seizures (extremely rare), short-term discomfort, pain, brief episodes of hypomania, cognitive change, hearing loss, impaired working memory, and inadvertent current induction in implanted devices such as pacemakers or defibrillators.
Diagnostic applications include
Measurement of the activity and function of specific brain circuits in humans.
Measurement of the connection between the primary cortex of the CNS and the peripheral nervous system (PNS): To evaluate damage related to past or progressive neurologic insult.
a. Neurologic conditions (potentially):
b. Alzheimer’s disease (AD),
c. Amyotrophic lateral sclerosis (ALS),
d. Persistent vegetative state,
e. Epilepsy,
f. Stroke-related disability,
g. Tinnitus,
h. Multiple sclerosis (MS),
i. Schizophrenia, and
j. Traumatic brain injury
k. Parkinson’s disease (PD): low-frequency stimulation may affect medication-associated dyskinesia whereas highfrequency stimulation may improve motor function.
l. Motor cortex:
m. Dominant side (tentative benefits),
n. Dorsolateral prefrontal cortex (variable results).
o. Motor symptoms. Less effective than electroconvulsive therapy though both appear to have utility.
p. Cerebellar stimulation: Potentially for levodopa-associated dyskinesia.
q. In psychiatry:
r. Anxiety disorders (panic disorder),
s. Obsessive-Compulsive Disorder (OCD): Most promising for the orbitofrontal cortex and the supplementary motor area
t. Autism,
u. Substance abuse,
v. Addiction,
w. Post-Traumatic Stress Disorder (PTSD),
x. Resistant major depressive disorder,
y. Mapping the functional connectivity between the cerebellum and other areas of the brain
Magnetic seizure therapy
Magnetic Seizure Therapy (MST) is under current investigation. It works by inducing seizures via magnetic fields.
Purported applications include:
a. Treatment-Resistant Depression (TRD).
b. Schizophrenia
c. Obsessive-Compulsive Disorder (OCD).
Pulsed (electromagnetic) field therapy
Pulsed electromagnetic field therapy (pEMFT), also known as low-field magnetic stimulation (lfMS), and tumor treating fields (TTF) uses electromagnetic fields. Several devices have been approved by the FDA.
Common applications include
a. Delayed and non-union fractures: The evidence of benefit is insufficient.
b. Depression.
c. Glioblastomas (brain tumors).
d. Knee osteoarthritis: Possible benefit for improved function but there is no evidence for pain alleviation.
e. Postoperative Pain.
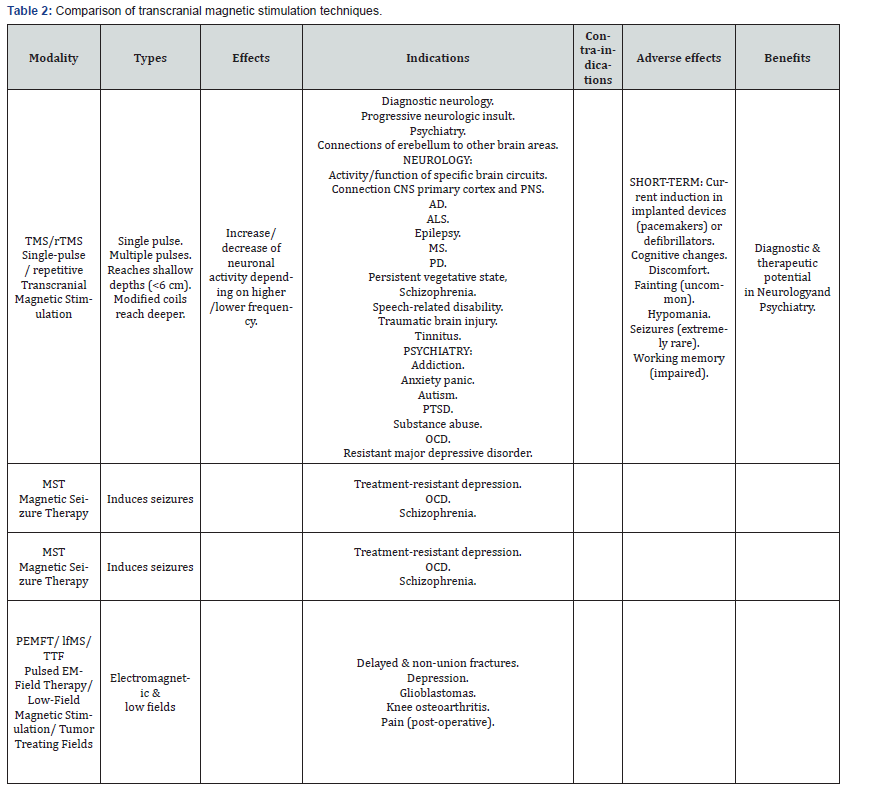
Table 2 provides a comparison of transcranial magnetic stimulation techniques.
Summary and Conclusion
The several known electrical and magnetic brain stimulations offer diagnostic and therapeutic features as well as research possibilities. This property has been used for diagnostic and therapeutic neurosurgical procedures. Electromagnetic stimulations have been separated into electrical (both direct and alternating currents) and magnetic stimulations. The former include transcranial electrical stimulation (with either direct or alternating current), electroconvulsive (or electroshock) therapy that remains controversial, functional (or neuromuscular) electrical stimulation for paralysis due to injury, vagus nerve stimulation and its transcutaneous variation. The latter include transcranial magnetic stimulation, magnetic seizure therapy, and pulsed electromagnetic field therapy (or low-field magnetic stimulation, or tumor treating fields). These several modalities were compared in their types, effects, indications, contraindications, adverse events, and benefits. Depending on the patient’s condition, the comparative Tables provided should guide the appropriate selection of procedure.
References
- Adee S (2012) Zap your brain into the fast track. New Scientist.
- Agarwal SM, Shivakumar V, Anushree Bose, Subramaniam V, Hema Nawani, et al. (2013) Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci 11(3): 118–125.
- Agid Y, Ahlskog E, Albanese A, Calne D, Chase T, et al. (1999) Levodopa in the treatment of Parkinson's disease: A consensus meeting. Mov Disord 14(6): 911-913.
- De Aguiar V, Paolazzi CL, Miceli G (2015) tDCS in post-stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex 63: 296-316.
- Aldini J, Giovanni (1804) Essai théorique et expérimental sur le galvanisme, avec une série d’expériences faites devant des commissaires de l’Institut National de France, et en divers amphithéâtres anatomiques de Londres. De l'Imprimerie de Fournier fils.
- Richard J (2001) The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association, (2nd edn). APA.
- Antal A, Alekseichuk I, Bikson M, Brockmoeller J, Brunoni AR, et al. (2017) Low intensity transcranial electric stimulation: Safety, ethical, legal, regulatory, and application guidelines. Clin Neurophysiol 128(9): 1774-1809.
- Appel CP (1972) Effect of electrosleep: Review of research. Goteborg Psychological Reports 2(1):1-24.
- Arias-Carrión O (2008) Basic mechanisms of rTMS: Implications in Parkinson's disease. Int Arch Med 1(1): 2.
- Bikson M, Datta A, Elwassif M (2009) Establishing safety limits for transcranial direct current stimulation, Clin Neurophysiol 120(6): 1033-1034.
- Bassett CA, Pawluk RJ, Pilla AA (1974) Acceleration of fracture repair by electromagnetic fields: A surgically noninvasive method. Ann N Y Acad Sci 238: 242-262.
- Bassett CA, Pawluk RJ, Pilla AA (1974) Augmentation of bone repair by inductively coupled electromagnetic fields. Science 184(4136): 575-577.
- Bassett CA, Mitchell SN, Norton L, Pilla A (1978) Repair of non-unions by pulsing electromagnetic fields. Acta Orthop Belg 44(5): 706-724.
- Beck M (2011) Using Electricity, Magnets for Mental Illness. The Wall Street Journal.
- Bennabi D, Pedron S, Haffen E, Monnin J, Peterschmitt Y (2014) Transcranial direct current stimulation for memory enhancement from clinical research to animal models. Front Syst Neurosci 8: 159.
- Berlim MT, Neufeld NH, Van den Eynde F (2013) Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res 47(8): 999-1006.
- Berrios GE (1997) The scientific origins of electroconvulsive therapy. Hist Psychiatry 8(29 pt 1): 105-119.
- Bersani FS, Minichino A, Enticott PG, Mazzarini L, Khan N, et al. (2013) Deep transcranial magnetic stimulation as a treatment for psychiatric disorders: A comprehensive review. Eur Psychiatry 28(1): 30-39.
- Blaine JD, Clark SM (1986) Report of the NIMH-NIH Consensus Development Conference on electroconvulsive therapy--statement of the Consensus Development Panel-statement of the Consensus Development Panel. Psychopharmacol Bull 22(2): 445-454.
- Boopalan PRJVC, Chittaranjan, Samuel B, Balamurugan, Ramadass, et al. (2009) Pulsed electromagnetic field (PEMF) treatment for fracture healing. Current Orthopaedic Practice 20(4): 423-428.
- Borckardt JJ, Bikson M, Frohman, H, Reeves ST, Datta A, et al. (2012) A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain 13(2): 112-120.
- Breggin PR (1979) Electroshock: Its brain-disabling effects. Springer.
- Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, et al. (2016) Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry 208(6): 522-531.
- Cacioppo JT, Tassinary LG, Berntson GG (2007). Handbook of psychophysiology (3rd edn.), Cambridge University Press.
- Cai PY, Bodhit A, Derequito R, Ansari S, Abukhalil F, et al. (2014) Vagus nerve stimulation in ischemic stroke: old wine in a new bottle. Front Neurol 5: 107.
- Carreno FR, Frazer A (2017) Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 14(3): 716-727.
- Cerletti U (1956) Electroshock therapy. In: AM Sackler (Eds.), The Great Physiodynamic Therapies in Psychiatry: A historical appraisal. New York: Hoeber-Harper, USA, pp.91-120.
- Chantraine A, Baribeault A, Uebelhart D, Gremion G (1999) Shoulder pain and dysfunction in hemiplegia: effects of functional electrical stimulation. Arch Phys Med Rehabil 80 (3): 328-331.
- Chou YH, Hickey PT, Sundman M, Song AW, Chen NK (2015) Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol 72(4): 432-440.
- Cook AW (1976) Electrical stimulation in multiple sclerosis. Hosp Pract 11(4): 51-58.
- Datta A, Bansal V, Diaz J, Patel J, Reato D, et al. (2009) Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2(4): 201-207.
- Delgado J (1986) Physical Control of the Mind: Toward a Psychocivilized Society. Harper.
- Dierckx B, Heijnen WT, Van Den Broek WW, Birkenhäger TK (2012) Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 14(2): 146-150.
- Dougall N, Maayan N, Soares-Weiser K, McDermott LM, McIntosh A (2015) Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database of Systematic Reviews.
- Edwards CA, Kouzani A, Lee KH, and Ross EK (2017) Neurostimulation devices for the treatment of neurologic disorders. Mayo Clin Proc 92(9): 1427-1444.
- Elsner B, Kugler J, Pohl M, Mehrholz J (2015) Transcranial direct current stimulation (tDCS) for improving aphasia in patients with aphasia after stroke. Cochrane Database Syst Rev 5.
- Elsner B, Kugler J, Pohl M, Mehrholz J (2016) Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev 3.
- Faria MA (2013) Violence, mental illness, and the brain - A brief history of psychosurgery: Part 2 - From the limbic system and cingulotomy to deep brain stimulation. Surg Neurol Int 4: 75.
- Feng WW, Bowden MG, Kautz S (2013) Review of transcranial direct current stimulation in poststroke recovery. Top Stroke Rehabil 20(1): 68-77.
- Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, et al. (2009) Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord 118(1-3): 215-219.
- Fink M (1984) Meduna and the origins of convulsive therapy. Am J Psychiatry 141(9): 1034-1041.
- Fink M, Taylor MA (2007) Electroconvulsive therapy: Evidence and Challenges. JAMA 298(3): 330-332.
- Fox D (2017) Can zapping the vagus nerve jump-start immunity? An experimental procedure is exposing links between nervous and immune systems. Scientific American.
- França C, de Andrade DC, Teixeira MJ, Galhardoni R, Silva V, et al. (2017) Effects of cerebellar neuromodulation in movement disorders: A systematic review. Brain Stimul 11(2): 249-260.
- Fregni F, Simon DK, Wu A, Pascual-Leone A (2005) Non-invasive brain stimulation for Parkinson's disease: A systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry 76(12): 1614-1623.
- Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A (2006) Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety 23(8): 482-484.
- Fregni F, Boggio PS, Nitsche, MA, Marcolin MA, Rigonatti SP, et al. (2006) Treatment of major depression with transcranial direct current stimulation. Bipolar Disord 8(2): 203-204.
- Friedberg J (1977) Shock treatment, brain damage, and memory loss: A neurological perspective. Am J Psychiatry 134(9): 1010–1014.
- Fymat AL (2017a) Epilepsy: A review, Journal of Current Opinions in Neurological Science 1(5): 240-254.
- Fymat AL (2017b) Neurological disorders and the blood-brain barrier: 1. Epilepsy, Journal of Current Opinions in Neurological Science 1(6): 277-293.
- Fymat AL (2017c) Parkinson's disease and other movement disorders: A review”, Journal of Current Opinions in Neurological Science 2(1): 316-343.
- Fymat AL (2018d) Neurological disorders and the blood-brain barrier: 2 Parkinson's disease and other movement disorders, Journal of Current Opinions in Neurological Science 2(1): 362-383.
- Fymat AL (2018e) Blood-brain barrier permeability and neurological diseases, Journal of Current Opinions in Neurological Science 2(2): 411-414.
- Fymat AL (2018f) Alzheimer's disease: A review, Journal of Current Opinions in Neurological Science 2(2): 415-436.
- Fymat AL (2018g) Regulating the brain's autoimmune system: The end of all neurological disorders? Journal of Current Opinions in Neurological Science 2(3): 475-479.
- Fymat AL (2018h) Alzheimer's disease: Prevention, delay, minimization, and reversal, Journal of Clinical Research in Neurology 1(1): 1-16.
- Fymat AL (2018i) Harnessing the immune system to treat cancers and neurodegenerative diseases. Journal of Clinical Research in Neurology 1(1): 1-14.
- Fymat AL (2018j) Is Alzheimer's an autoimmune disease gone rogue? Journal of Clinical Research in Neurology 2(1): 1-4.
- Fymat AL (2018k) Dementia treatment: Where do we stand? Journal of Current Opinions in Neurological Science 3(1): 1-3.
- Fymat AL (2018l) On dementia and other cognitive disorders, Journal of Clinical Research in Neurology 1(2): 1-14.
- Fymat AL (2018m) Is Alzheimer's a runaway autoimmune disease? and how to cure it? Newsletter European Union Academy of Sciences Annual Report (2018) and Proceedings of the European Union Academy of Sciences (2018) Newsletter, pp. 379-383.
- Fymat AL (2019a) Dementia: A review, Journal of Clinical Psychiatry and Neuroscience 1(3): 27-34.
- Fymat AL (2019b) The Pathogenic Brain, Journal of Current Opinions in Neurological Science 3(2): 669-671.
- Fymat AL (2019c) On the pathogenic hypothesis of neurodegenerative diseases”, Journal of Clinical Research in Neurology 2(1): 1-7.
- Fymat AL (2019d) Dementia with Lewy bodies: A review, Journal of Current Opinions in Neurological Science 4(1): 15-32.
- Fymat AL (2019e) Our two interacting brains – Etiologic modulations of neurodegenerative and gastroenteric diseases, Journal of Current Opinions in Neurological Science 4(2): 50-54.
- Fymat AL (2019f) What do we know about Lewy body dementias? Journal of Psychiatry and Psychotherapy.
- George MS, Post RM (2011) Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry 168(4): 356-364.
- Griffin XL, Costa ML, Parsons N, Smith N (2011) Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults. Cochrane Database Syst Rev 4: CD008471.
- Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, et.al (2016) Cerebellar transcranial direct current stimulation (ctDCS): A Novel Approach to Understanding Cerebellar Function in Health and Disease. Neuroscientist 22(1): 83-97.
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, et al. (2012) A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol 123(5): 858-882.
- Groves DA, Brown VJ (2005) Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience & Biobehavioral Reviews 29(3): 493-500.
- Guridi J, Lozano AM (2011) A brief history of pallidotomy. Neurosurgery 41: 1169-1180.
- Iwanovsky A, Dodge CH (1968) Electrosleep and electroanesthesia: Theory and clinical experience. Foreign Science Bulletin 4(2): 1-64.
- Guyton and Hall (2015) Textbook of Medical Physiology, John Hall, 13th (edn.), Elsevier Health Sciences, Netherlands.
- Han P, Frei MG, Osorio I (1996) Probable mechanisms of action of vagus nerve stimulation in humans with epilepsy: Is a window into the brain? Epilepsia 37(5 Suppl): 83s.
- Harty S, Robertson IH, Miniussi C, Sheehy OC, Devine CA, et al. (2014) TranscranIal direct current stimulation over right dorsolateral prefrontal cortex enhances error awareness in older age. J Neurosci 34(10): 3646-3652.
- Holtzheimer PE, Mayberg HS (2010) Deep brain stimulation for treatment-resistant depression. Am J Psychiatry 167(12): 1437-1444.
- Howland, RH (2014) Vagus nerve stimulation. Curr Behav Neurosci Rep 1(2): 64-73.
- Jansen JM, Daams JG, Koeter MW, Veltman,DJ, van den Brink W, et al. (2013) Effects of non-invasive neurostimulation on craving: A meta-analysis. Neurosci Biobehav Rev 37(10 Pt 2): 2472-2480.
- Katsoulaki M, Kastrinis A, Tsekoura M (2017) The effects of anodal transcranial direct current stimulation on working memory. Ge Ne Dis Advances in Experimental Medicine.
- Katzenschlager R, Lees AJ (2002) Treatment of Parkinson's disease: Levodopa as the first choice. J Neurol 249(Suppl 2): II19–24.
- Kavirajan HC, Lueck K, Chuang K (2014) Alternating current cranial electrotherapy stimulation (CES) for depression. Cochrane Database Syst Rev 7: CD010521.
- Kirsch DL, Nichols F (2013) Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. The Psychiatric Clinics of North America 36(1): 169-176.
- Krack P, Martinez-Fernandez R, del Alamo M, AObeso JA (2017) Current applications and limitations of surgical treatments for movement disorders. Mov Disord 32(1): 36-52.
- Kralj A, Bajd T, Turk R (1988) Enhancement of gait restoration in spinal injured patients by functional electrical stimulation. Clin Orthop Relat Res 34-43.
- Krishnan S, Pisharady KK (2017) Surgical treatment of levodopa-induced dyskinesia in Parkinson's disease, Ann Indian Acad Neurol 20(3): 199-206.
- Kuo HL, Bikson M, Datta A, Minhas P, Paulus W, et al. (2013) Comparing Cortical Plasticity Induced by Conventional and High-Definition 4 × 1 Ring tDCS: A Neurophysiological Study. Brain Stimul 6(4): 644-648.
- Lefaucheur JP (2009) Treatment of Parkinson's disease by cortical stimulation. Expert Rev Neurother 9(12): 1755-1771.
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, et al. (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiol 125(11): 2150-2206.
- Lefaucheur JP, Antal, A, Ayache SS, Benninger DH, Brunelin J, et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation. Clinical Neurophysiology 128(1): 56-92.
- Li H, Wang J, Li C, Xiao Z (2014) Repetitive transcranial magnetic stimulation for panic disorder in adults. Cochrane Database Syst Rev 9: CD009083.
- Liebetanz D, Koch R, Mayenfels S, König F, Paulus, W, et al. (2009) Safety limits of cathodal transcranial direct current stimulation in rats. Clinical Neurophysiology 120(6): 1161-1167.
- Lipsman N, Sankar T, Downar J, Kennedy SH, Lozano AM, et al. (2014) Neuromodulation for treatment-refractory major depressive disorder. CMAJ 186(1): 33-39.
- Luber B, Lisanby SH (2014) Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS)". NeuroImage 85 Pt 3(3): 961-970.
- Luedtke K, Rushton A, Wright C, Geiss B, Juergens TP, et al. (2012) Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: A systematic review and meta-analysis. Clin J Pain 28(5): 452-461.
- Machado S, Bittencourt J, Minc D, Portella CE, Velasques B, et al. (2008) Therapeutic applications of repetitive transcranial magnetic stimulation in clinical neurorehabilitation. Funct Neurol 23(3): 113-122.
- Malhi GS, Tanious M, Berk M (2012) Mania: Diagnosis and treatment recommendations. Curr Psychiatry Rep 14(6): 676-686.
- Marangell LB, Martinez M, Jurdi RA, Zboyan H (2007) Neurostimulation therapies in depression: A review of new modalities. Acta Psychiatrica Scandinavica 116(3): 174-181.
- Markov MS (2007) Expanding the use of pulsed electromagnetic field therapies. Electromagn Biol Med 26(3): 257-274.
- Martiny K, Lunde M, Bech P (2010) Transcranial low voltage pulsed electromagnetic fields in patients with treatment-resistant depression. Biol Psychiatry 68(2): 1633-1639.
- Miller MC (2012) Magnetic stimulation: A new approach to treating depression. Harvard Health Publications.
- Moe JH, Post HW (1962) Functional electrical stimulation for ambulation in hemiplegia. J Lancet 82: 285-288.
- Mondino M, Bennabi D, Poulet E, Galvao F, Brunelin J, et al. (2014) Can transcranial direct current stimulation alleviate symptoms and improve cognition in psychiatric disorders? World J Biol Psychiatry 15(4): 261-275.
- Mutz J, Vipulananthan V, Carter B, Hurlemann R, H Y Fu C, et al. (2019) Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: Systematic review and network meta-analysis. BMJ 364: 11079.
- National Institute for Health and Clinical Excellence (2014) Transcranial magnetic stimulation for treating and preventing migraine.
- National Institute for Health and Clinical Excellence (2016). Functional electrical stimulation for drop foot of central neurologic origin.
- National Institutes of Health (2013) Magnetic Seizure Therapy (MST) for treatment-resistant depression, schizophrenia, and obsessive-compulsive disorder.
- Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology 527(3): 633-639.
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, et al. (2003) Safety criteria for transcranial direct current stimulation in humans. Clinical Neurophysiology 114(11): 2220-2222.
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, RothwellJC, et al. (2003) Level of action of cathodal DC polarization induced inhibition of the human motor cortex. Clinical Neurophysiol 114(4): 600-604.
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, et al. (2008) Transcranial direct current stimulation: State-of- the-art 2008. Brain Stimul 1(3): 206-223.
- Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A (2009) Treatment of depression with transcranial direct current stimulation: A Review. Exp Neurol 219(1): 14-19.
- Nowak DA, Bösl K, Podubeckà J, Carey JR (2010) Noninvasive brain stimulation and motor recovery after stroke. Restor Neurol Neurosci 28(4): 531–544.
- Oberman LM, Enticott PG, Casanova MF, Rotenberg A, Pascual-Leone A, et al. (2016) Transcranial magnetic stimulation in autism spectrum disorder: Challenges, promise, and roadmap for future research. Autism Research 9(2): 184-203.
- Panebianco M, Rigby A, Weston J, Marson AG (2015) Vagus nerve stimulation for partial seizures. Cochrane Database Syst Rev (4): CD002896.
- Parent A (2004) Giovanni Aldini: From animal electricity to human brain stimulation. Can J Neurol Sci 31(4): 576-584.
- Parent A (2004) Aldani's essay on galvanism. The Canadian Journal of Neurological Sciences 31(4): 576-584.
- Pascual-Leone A, Davey N, Rothwell J, Wassermann EM, Puri BK (2002) Handbook of Transcranial Magnetic Stimulation.
- Pedron S, Monnin J, Haffen E, Sechter D, Van Waes V (2013) Repeated transcranial direct current stimulation prevents abnormal behaviors associated with abstinence from chronic nicotine consumption. Neuropsychopharmacology 39(4):981-988.
- Penfield W (1974) Speech and Brain Mechanisms. New York: Atheneum.
- Pereira S, Mehta S, McIntyre A, Lobo L, Teasell RW (2012) Functional electrical stimulation for improving gait in persons with chronic stroke. Top Stroke Rehabil 19(6): 491-498.
- Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, et al. (2014) Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev 11: CD010820.
- Popovic MR, Thrasher TA (2004) Neuroprostheses in Encyclopedia of Biomaterials and Biomedical Engineering. GE Wnek GL Bowlin (Eds). Marcel Dekker, Inc, USA, 2: 1056-1065.
- Popovic MR, Thrasher TA, Zivanovic P, Takaki M, Hajek V (2005) Neuroprosthesis for retraining reaching and grasping functions in severe hemiplegic patients. Neuromodulation 8(1): 58-72.
- Popovic D, Sinkjaer T (2012) Control of movement for the physically disabled: Control for rehabilitation technology, Springer Science & Business Media.
- Popovic MR, Masani K, Micera S (2015) Chapter 9- Functional Electrical Stimulation Therapy: Recovery of function following spinal cord injury and stroke, in Neurorehabilitation Technology (2nd edn), Z Rymer, T Nef, V Dietz, (Eds.), Springer Science Publishers, Germany.
- Poreisz C, Boros K, Antal A, Paulus W (2007) Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 72(4-6): 208-214.
- Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, et al. (2003) Limitations of current Parkinson's disease therapy. Ann Neurol 53(Suppl 3): S3-12.
- Read J, Bentall R (2010) The effectiveness of electroconvulsive therapy: A literature review. Epidemiol Psichiatr Soc 19(4): 333-347.
- Riehl M (2008) TMS Stimulator Design. In Wassermann EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH (Eds.), Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press, England. p. 13-23.
- Rossini PM, Rossi S (2007) Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology 68(7): 484-488.
- Rudorfer MV, Henry ME, Sackheim HA (1997) Electroconvulsive therapy. In A Tasman, JA Lieberman (eds.), Psychiatry (2nd ed). Chichester: John Wiley, USA pp. 1535-1556.
- Rudorfer MV, Henry ME, Sackeim, HA (2003) Electroconvulsive therapy. In A Tasman, J Kay, JA Lieberman (eds). Psychiatry (Second Edition). Chichester: John Wiley & Sons Ltd, USA. pp. (1865–1901).
- Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, et al. (2013) Transcranial Current Brain Stimulation; Models and Technologies. IEEE Transactions on Neural Systems and Rehabilitation Engineering 21(3): 33-45.
- Sabbah HN (2011) Electrical vagus nerve stimulation for the treatment of chronic heart failure. Clevel Clin J Med 78(1): S24-29.
- Salat D, Tolosa E (2013) Levodopa in the treatment of Parkinson's disease: Current status and new developments. J Parkinson's Dis 3(3): 255-269.
- Salote C, Turi Z, Paulus W, Antal S (2013) Combining functional magnetic resonance imaging and transcranial electrical stimulation. Front Hum Neurosci 7: 435.
- Schwedt TJ, Vargas B (2015) Neurostimulation for treatment of migraine and cluster headache. Pain Medicine 16(9): 1827-1834.
- Selimbeyoglu A, Parvizi M (2010) Electric stimulation of the human brain: Perceptual and behavioral phenomena reported in the old and the new literature. Frontiers in Human Neuroscience 4(46): 46.
- Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, et al. (2018) Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia: A systematic review. Ann Intern Med 168(6): 414-421.
- Shin SS, Dixon CE, Okonkwo DO, Richardson RM (2014) Neurostimulation for traumatic brain injury. J Neurosurg 121(5): 1219-1231.
- Shiozawa P, Silva ME, Carvalho TC, Cordeiro Q, Brunoni AR, et al. (2014) Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: A systematic review. Arquivos de Neuro-Psiquiatria 72(7): 542-547.
- Sienaert P, Dhossche DM, Vancampfort D, De Hert M, Gazdag G (2014) A clinical review of the treatment of catatonia. Front Psychiatry 5: 1811.
- Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, et al. (2010) Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 133(9): 2565-2577.
- Sparing R, Mottaghy FM (2008) Noninvasive brain stimulation with transcranial magnetic or direct current stimulation: From insights into human memory to therapy of its Methods 44(4): 329-337.
- Street T, Taylor P, Swain I (2015) Effectiveness of functional electrical stimulation on walking speed, functional walking category, and clinically meaningful changes for people with multiple sclerosis. Arch Phys Med Rehabil 96(4): 667-672.
- Tharyan P, Adams CE (2005) In Tharyan, Prathap (eds.). Electroconvulsive therapy for schizophrenia. The Cochrane Database of Systematic Reviews (2): CD000076.
- Torres F, Villalon E, Poblete P, Moraga-Amaro R, Linsambarth S, et al. (2015) Retrospective evaluation of deep transcranial magnetic stimulation as add-on treatment for Parkinson's disease. Front Neurol 6: 210.
- Utz KS, Dimova, Violeta O, Kerkhoff G (2010) Electrified minds: Transcranial direct currents stimulation and galvanic vestibular stimulation as methods of non-invasive brain stimulation in neuropsychology: A review of current data and future implications. Neuropsychologia 48(10): 2789-810.
- van Belkum SM, Bosker FJ, Kortekaas R, Beersma DG, Schoevers RA (2016) Treatment of depression with low-strength transcranial pulsed electromagnetic fields: A mechanistic point of view. Prog Neuropsychopharmacol Biol Psychiatry 48(10): 137-143.
- van den Noort M, Lim S, Bosch P (2014) Recognizing the risks of stimulation of the brain. Science 346(6215): 1307.
- van Dun K, Bodranghien FCAA, Mariën P, Manto MU (2016) Transcranial direct current stimulation of the cerebellum: Where do we stand in 2016? Technical issues and critical review of the literature. Front Hum Neurosci 10: 199.
- van Dun K, Bodranghien F, Manto M, Mariën P (2017) Targeting the cerebellum by noninvasive neurostimulation: A review. Cerebellum 16(3): 695-741.
- Vercammen A, Rushby J, Loo C, Short B, Weickert CS, and Weickert TW (2011) Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophrenia Research 131(1-3): 198-205.
- Viganò A, d Elia TS, Sava SL, Auvé M, De Pasqua V, et al. (2013) Transcranial Direct Current Stimulation (tDCS) of the visual cortex: A proof-of- concept study based on interictal electrophysiological abnormalities in migraine. J Headache Pain 14(1): 23.
- Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, et al. (2003) Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol 53(5): 558-569.
- Yang C, Guo Z, Peng H, Xing G, Chen H, et al. (2018) Repetitive transcranial magnetic stimulation therapy for motor recovery in Parkinson's disease: A meta-analysis. Brain Behav 8(11): e01132.
- Yokol Y, Sumiyashi T (2015) Application of transcranial direct current stimulation to psychiatric disorders: Trends and perspectives, Neuropsychiatric Electrophysiology 1(10).
- Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F (2010) Noninvasive brain stimulation with low-intensity electrical currents: Putative mechanisms of action for direct and alternating current stimulation. Neuroscientist 16(3): 285-307.






























