Rtms - A Promising Non-Pharmacologic Therapy for Migraines
Mahmoud Okasha S*, Nathan Skoller and Martha Koo
Associate clinical professor, Yale University Medical School,USA
Submission: April 09, 2019; Published: May 06, 2019
*Corresponding author: Mahmoud OKasha, Associate clinical professor, Yale University Medical School, 200 WEST Town ST Norwich CT, USA
How to cite this article: Mahmoud Okasha S, Nathan Skoller, Martha Koo. Rtms - A Promising Non-Pharmacologic Therapy for Migraines. Open Access J Neurol Neurosurg. 2019; 10(5): 555797. DOI: 10.19080/OAJNN.2019.10.555797.
Abstract
Non-pharmacologic treatment modalities for migraine are in demand. High Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) was investigated as a potential prophylactic migraine treatment. Ten subjects suffering from chronic migraine with and without aura were followed over 7 months. Beginning at month 3, patients received 25 sessions of interventional rTMS treatment using a Neuro star TMS device. 1600 pulses per session were administered to the left dorsolateral prefrontal cortex at 90% of the motor threshold. 20Hz two-second pulse trains were separated by 30 second rest intervals. When comparing baseline to post-treatment measurements, a statistically significant reduction in the frequency of attacks, reduction in disability, and improvement in quality of life was shown. Reduction in severity of attacks trended toward significance. These results confirm previous findings by others that rTMS may have a role in migraine prevention.
Keywords: Transcranial Magnetic stimulation; Cortical Spreading; Migraine; Migraine severity scale(MIGSEV); Migraine severity assessment( MIDAS); Qualite de vie(QVM)
Introduction
Migraine affects an estimated 20% of the population and approximately three times more women than men [1]. In the United States alone, more than 60 million individuals suffer with migraine. While pharmacological treatment options are available, demand for non-pharmacological modalities exist [2,3]. TMS (Transcranial Magnetic Stimulation), proven to relieve neuropathic pain, has been hypothesized as a treatment modality to reduce the frequency of migraine attacks [2, 4-12].
Currently the most likely mechanism leading to migraines with or without aura is trigeminovascular activation and Cortical Spreading Depression (CSD) The trigeminal system consists of nerve endings on meningeal vessels. Activation of this system during a migraine attack releases substance such as calcitonin and substance P that cause vasodilatation, leading to nociceptive neural transmission via the trigeminal nucleus and resulting pain+ [3,4,13,17,22,25, 27-30].
Medications that are effective in migraine prevention have been shown to inhibit CSD in animal models [20]. Similarly, rTMS has also been shown in animal models to inhibit CSD [12]. Using high frequency rTMS over the DLPFC, in 2007 Brighina et al, showed a significant reduction in migraine attacks, drug consumption, and headache index [13]. Several subsequent studies demonstrated mixed efficacy results. [4,5,13,15,]. Without a clear consensus on the role of rTMS for migraine prevention, more research is needed, prompting our study.
Materials and Methods
We chose to investigate rTMS for migraine treatment using an interrupted time-series study design. Ten subjects were followed for 7 months and received a 6-week rTMS intervention after the third month. Subjects were asked to record every migraine attack they experienced using a provided migraine log. Log entries included a MIGSEV scale, abortive medicines taken, and other basic details describing the length and timing of the of the migraine attack. The MIGSEV is a validated four-question scale used to assess migraine severity. Every month subjects were also asked to complete one Midas scale and one QVM scale used to measure disability and quality of life respectively.
The Neuro star TMS Therapy system was utilized for rTMS therapy in this study. Subjects received TMS treatment at one of 2 study locations. 5 rTMS treatments sessions were performed over 6 weeks. 20 of these treatment sessions were completed within the first four weeks. The remaining five sessions were completed over the fifth and sixth weeks. A maximum of one treatment was performed per day. Subjects were treated on weekdays only. Each treatment session consisted of 1600 pulses, or 40 trains, with 30-second resting intervals between trains. Each train was administered to the DLPFC at 20HZ for 2 seconds at an intensity set at 90% of the subject’s measured motor threshold. To accommodate subject’s sensitivity to treatment, during the first 3 treatments session, a reduction of pulse intensity to a minimum of 80% of motor threshold was considered acceptable upon subject request.
The study population included ten adults age 18 or older with an average age of 51.3 years old. Nine of the ten participants were female. All subjects met criteria for diagnosis of chronic migraine, migraine with Aura, or migraine without Aura using the International Headache Society Classification of Headache Disorders (ICHD II). Each participant was required to have had at least one migraine per month in the six months preceding enrollment. Individuals with ferromagnetic material implanted in their heads or within twelve inches of the treatment coil were excluded from the study. Pregnant subjects or subjects with implants controlled by physiological signals were also excluded from the study. Subjects receiving pharmacological therapy for migraine treatment prior to enrollment were encouraged to continue treatment as usual without significant medication regimen changes in order to avoid confounding effects.
Results
Pre-Therapy was defined as month 1 of subject data. Post Therapy was defined as month 5 of subject data. Months were analyzed using one-tailed Wilcoxon signed rank tests to evaluate if post therapy data showed significant improvement at endpoints when compared to pre-therapy data. An alpha level less than 0.05 was used to determine statistical significance. One subject’s month 4 data were utilized as post-therapy data in place of month 5, as the patient was lost to follow-up after month 4.
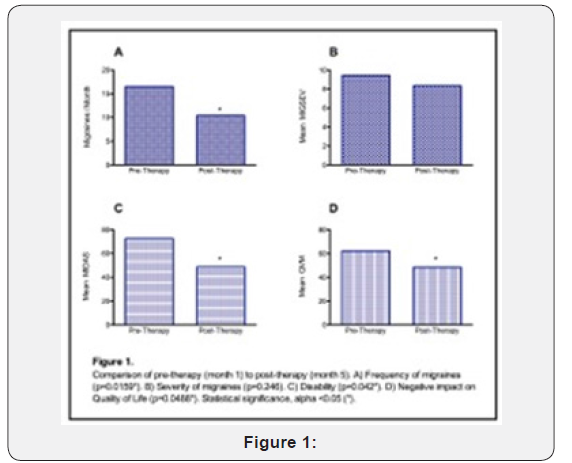
Figure 1 illustrates comparisons of pre and post therapy data of primary and secondary endpoints amongst all subjects. Frequency of migraines was significantly reduced post-therapy when compared to the pre-therapy period (p = 0.0159). There was no statistically significant reduction in severity of migraines between pre and post therapy periods (p=0.246). Disability was significantly reduced post-therapy when compared to the pretherapy period (p=0.042). Negative impact on quality of life was significantly reduced post-therapy when compared to the pretherapy period (p=0.0488).
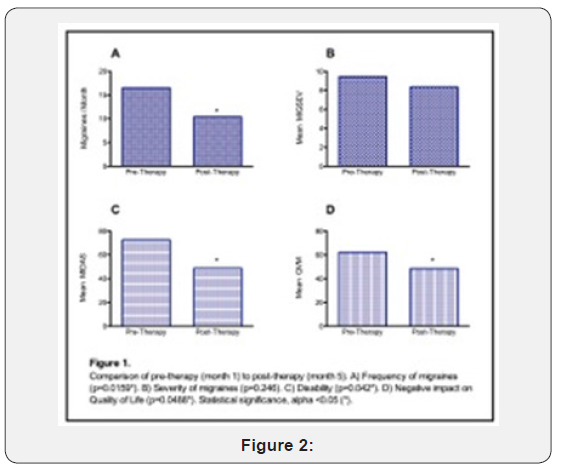
Figure 2 illustrates percent efficacy effects amongst study subjects who exhibited measured beneficial response to therapy. 70% of patients showed a 56.5% mean reduction in the frequency of migraines after intervention. 50% of patients showed a 42.3% mean reduction in the severity of migraine attacks. 60% of patients reported a 76.6% mean reduction in disability. Two patients were not included in our analysis: one patient who discontinued participation before receiving any rTMS therapy in order to pursue an alternate form of migraine therapy and one patient who completed the rTMS therapy but did not provide any survey data.
Discussion
High frequency rTMS (10-20Hz) as opposed to low frequency (1Hz) has shown improvement in the prophylaxis of migraines in some studies while failing to show improvement in others [19]. In a randomized study of 11 patients, 6 receiving active treatment and 5 receiving sham treatment,12 sessions of rTMS were delivered on alternative days to the left dorsolateral prefrontal cortex using a protocol delivering 400 pulses of 20 HZ at 90%of motor threshold, showing significant improvement of outcome measures in active versus sham treatment [13]. Another study using 10Hz protocol failed to reproduce these findings [22]. In a third randomized study, patients were treated with 3 alternate-day sessions of 600 pulse at 10 HZ to the right abductor digiti-minimi locus of the motor cortex, and improvement in outcome measures were shown in the group receiving active versus sham treatment. The differences in these results may be due to the different protocols. Amongst these studies, the type of patients, stimulation parameters, number of pulses and site of [4] stimulation vary. Our study differs from these previous trials in that a longer treatment course was used. In the treatment of other disorders, such as fibromyalgia, chronic pain, and major depressive disorder more treatment sessions resulted in improved results [6,7,23]. This knowledge was the basis for our choice to utilize a longer versus a shorter treatment course. Migraines are related to these other disorders as they all lead to altered excitability of cortical and subcortical structures secondary to dysfunction of neuronal ion channels.
While significant improvement of migraine symptoms were demonstrated, lack of sham treatment and small sample size are limiting factors to the generalizability of our results. Difficulty collecting complete data sets from participants over seven months may have impacted results, given all post-therapy data was not able to be utilized in analysis. However, incomplete post-therapy data not included in our results appeared without formal analysis to trend in the direction of our findings.
Our results suggest that rTMS therapy may be an effective prophylactic treatment as well as a non-medication treatment intervention that reduces disability caused by migraines and improves quality of life in migraine sufferers. Study conditions mimicked real-world clinical treatment scenarios, as patients did not alter pre-existing pharmacotherapy regimens throughout the course of the study. Women comprised the majority of our study population, which reflects the prevalence of migraines across gender in the United States. Additionally, as study participants logged migraines on a daily basis, recall bias was kept to a minimum during data collection.
rTMS was shown to successfully prevent migraine attacks in our study indicating there is clear need for large doubleblind randomized control trials. Future trials should investigate migraine prophylaxis via rTMS with longer treatment courses similar to the protocol used in our study. The future of migraine treatment and/or prophylaxis is likely to include rTMS therapy and further research remains necessary.
Conclusion
These results demonstrate that a course of 25 sessions of rTMS delivered over the DLPFC at 20Hz with 1600 pulses per session at 90% of motor threshold reduces migraine frequency, reduces disability from migraines, and increases quality of life in migraineurs, confirming similar findings in previous studies.
Acknowledgement
The authors are thankful to Neurostar corporation for providing free Senstars.
References
- Lipton RB, Pearlman SH (2010) Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics 7(2): 204-212.
- Jen-Kun Cheng (2010) repetitive Transcranial stimulation -an alternative treatment for chronic refractory pain Acta Anesthesiologic Taiwanica 30(2):137-144.
- Misra UK, Kalita J, Bhoi SK (2013) high rate TMS in migraine prophylaxis, a randomized placebo controlled study J Neurol 260(11): 2793-27801.
- Misra UK, Kalita J, Bhoi SK (2012) high frequency repetitive TMS is effective in Migraine prophylaxis an open label study. Neurol Res 34(6): 547-551.
- Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, et al. (2004) rTMS of the prefrontal cortex in the treatment of chronic Migraine a pilot study J of neurological sciences 227(1): 67-71.
- (2011) Conforto A Effects of rTMS in chronic Migraine a pilot study. 94.
- Boyer L, Dousset A, Roussel P, Dossetto N, Cammilleri S (2014) rTMS in Fibromyalgia a randomized trial evaluating QUL and its brain metabolic substrates. Neurology 182(14): 1231-1238.
- Fricova J, Klírová M, Masopust V, Novák T, Vérebová K (2013) rTMS in the treatment of Chronic orofacial pain. Physiology Res 62 Suppl 1: S125-34.
- George MS post RMS (2011) daily left prefrontal cortex rTMS for acute treatment of medication resistant depression Am J Psychiatry 168 (4): 356-364.
- Holland PR (2009) Modulation of trigemino vascular processing: Novel insight into primary headaches disorders. Cephalgia 29 suppl 3: 1-6.
- Schwedt TJ, Vargas B (2015) Neuro stimulation for treatment of Migraine and cluster headache. Pain med 16(9): 1827-1834.
- Brighina F (2013) Brain stimulation in Migraine Brain stimulation in Migraine Hand book of clinical neurology. Handb Clin Neurol 116: 585- 598.
- Andrews Charles (2012) the evolution of a migraine attack- a review of recent evidence Headaches Currents. 53(12): 413-419.
- Bruno Colombo, Dacia Dalla Libera, Gloria Dalla Costa, Giancarlo Comi (2013) Refractory migraine: the role of the physician in assessment and treatment of a problematic disease. Neurological science 34 (suppl1): S109-S112.
- Barr MS, Farzan F, Davis KD, Fitzgerald PB, Daskalakis ZJ (2013) Measuring Gabaergic inhibitory activity with TMS EEG and its potential application for chronic pain. J Neuroimmune Pharmacol 8(3): 535-546.
- Brigo F, Storti M, Nardone R, Fiaschi A, Bongiovanni LG, et al. (2012) Transcranial Magnetic stimulation of visual cortex in migraine patients: a systematic review with Meta-analysis. J headache pain 13(5): 339-349.
- Eli Soto (2012) Central Neurostimulation techniques for Primary headaches techniques in regional anesthesia and pain management 164: 47-56.
- Weatherall MW (2015) the diagnosis and treatment of chronic Migraine. Ther Adv Chronic Dis 6(3): 115-123.
- Borckardt JJ, Reeves ST, Frohman H, Madan A, Jensen MP, et al. (2011) Fast left prefrontal rTMS acutely suppresses analgesic effects of perceived controllability on the emotional component of pain experience. Pain 152(1): 182-187.
- Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D (2014) rTms stimulation induced analgesia depends on NMDA glutamate receptors. Pain 155(3): 598-605.
- T Sasso D Elia Quadruple (2013) rTMS on visual cortex for chronic migraine prevention Abstracts of the 2013 International headache congress. 49-50
- Paemeleire K, Louis P, Magis D, Vandenheede M, Versijpt J, et al. (2015) diagnosis pathophysiology and management of chronic migraine: a proposal of the Belgian headache Society. Acta Neurol Belg 115(1): 1-17.
- Jean Pascal Le Faucheur, Isabelle Ménard-Lefaucheur, Colette Goujon, Yves Keravel, Jean-Paul Nguyen (2011) predictive Value of rTMS in the identification of responders to Epidural Motor Cortex stimulation therapy for pain. J of Pain12 (10): 1102-1111.
- Mhalla A, Baudic S, Ciampi de Andrade D, Gautron M, Perrot S, et al. (2011) long term maintenance of the analgesic effects of transcranial Magnetic stimulation in fibromyalgia 152(7): 1478-1485.
- Badawy RA, Loetscher T, Macdonell RA, Brodtmann A (2012) Migraine Cortical excitability and neurology: insights into the pathophysiology Functional neurol 27(3): 131-145.Lefaucheur JP (2008) Use of Repetitive transcranial magnetic stimulation in pain Relief. Expert Rev Neurother 8(5): 799-808.
- David Borsook (2012) neurological disease and pain. Brain 135(2): 320-344.
- Lorenz J, Minoshima S, Casey KL (2003) keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126 (Pt5): 1079-1091.
- O Reardon JP, Fontecha JF, Cristancho MA, Newman S (2007) unexpected reduction in migraine and psychogenic headaches following rTMS treatment for Major depression: a report of two cases. CNS Spectr 12 (12): 921-925.
- Teepker M, Hötzel J, Timmesfeld N, Reis J, Mylius V, et al. (2010) low frequency rTMS of the Vertex in the prophylactic treatment of Migraine. Cephalgia 30(2): 137-144.
- Teepker M, Hötzel J, Timmesfeld N, Reis J, Mylius V, et al. (2010) low frequency TMS of the vertex in the prophylactic treatment of Migraine. Cephalgia 30(2): 137-144.






























