Multiple Sclerosis Infected by Human Lymphotropic Virus Type-1 (HTLV-1)-Associated Myelopathy/Tropical Spastic Paraparesis T-Cell (HAM/TSP); A Case Report
Saeed Shahbeigi1*, Mojdeh Bolouchian2, Altintas A3, Gharegozli K1 and Hossein Pakdaman1
1Department of Neurology, Shahid Beheshti Medical University Research Center, Iran
2Department of Medical Sciences, Islamic Azad university, Iran
3Department of Neurology, Koc University, Turkey
Submission: January 17, 2019; Published: March 13, 2019
*Corresponding author: Shahbeigi Saeed, Shahid Beheshti Medical University Research Center, Tehran, Iran
How to cite this article: Sibhi G, Priyamvadha K, Abha V, Vidya B. Dorsal Intradural Extramedullary Epidermoid in a One Year Old Child - A Case Report and Review of Literature . Open Access J Neurol Neurosurg. 2019; 10(1): 555779. DOI: 10.19080/OAJNN.2019.10.555779.
Abstract
Multiple Sclerosis (MS) is a chronic neurologic disease that affects female more than male and estimated as the major neurologic cause of disability in adult between 20 to 40 years old. Around 2.5 million people have been suffered from MS in all parts of the world. In addition to that, Human lymphotropic virus type-1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis T-cell (HAM/TSP) is a severe chronic neurological disease caused by Human T-cell lymphotropic virus type-1 (HTLV-1). HAM/TSP is a neurological disease that causes disability as well. In endemic area, HAM/TSP is one of the major disorders that mimics primary progressive type of MS.
Simultaneous involvement of MS and HAM/TSP is a rare condition in the medical literature. In our knowledge, four Brazilian patients have been reported with MS infected by HTLV-I [1]. We are presenting a case who has had neurological symptoms for 16 years. She was diagnosed as MS and has been given many types of Disease Modifying Treatments (DMTs), but finally due to the positivity of anti- HTLV1- Ab in both blood and CSF, as well as positive HTLV1 DNA- PCR, she was diagnosed as MS infected by HTLV1 as well.
In this case report, we aim to discuss our case in the light of current knowledge on HAM/TSP and share our treatment approach.
Keywords: Multiple sclerosis; myelopathy; HTLV-1; tropical spastic paraparesis
Abbrevations: MS: Multiple Sclerosis; DMT: Disease Modifying Treatments; CSF: Cerebrospinal Fluid; VEPs: Visual Evoked Potentials; PBMC: Peripheral Blood Mononuclear Cells
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1) causes HTLV-1-associated myelopathy (HAM) [2]. Asymptomatic carrier with high peripheral blood mononuclear cell (PBMC) HTLV-1 proviral load more than one percent is at high risk of suffering of myelopathy. This health condition is more dominant in females older than 40 and anticipated 10-20 million people in the world are infected with HTLV-1[3]. The prevalence of HTLV-I infection between Mashhad people that is located in North East of Iran is 3% and among blood donators is 0.44% [4]. HTLV-I global prevalence is perhaps undervalued since it remnants uninvestigated in several large populated areas [2]. Most HTLV-I infected persons stay asymptomatic throughout their lives, whereas 2-5% could progress to adult T-cell leukemia/ATL and 0.25-3.8% can change into HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [5]. High endemic zones include Japan, Caribbean area, South America, Africa and North East portion of Iran [6].
The pathogenesis of TSP/HAM is not well defined and some features like viral and host factors such as the proviral load and the cellular immune reaction, act a main role in disease development. HTLV-1 viral load in Cerebrospinal Fuid (CSF) and Peripheral Blood Mononuclear Cells (PBMCs) in people with HAM/TSP is higher than those HTLV-1–infected individuals without any obvious neurologic disease. Moreover, higher HTLV-1 viral load is related to more rapid development of motor disability [7].
The pathological and imaging studies indicate both the brain and spinal cord could become involved in HTLV-1 infection. Pyramidal tract destruction with myelin and axonal loss, especially in the lower thoracic spinal cord are mainly detected. In early phase of disease, the dominant cells are nearly CD4+ and in the last phases mostly are CD8+. In patients with HAM–TSP, HTLV-1 infects mostly C-C chemokine receptor type4 (CCR4), that was found exclusively on memory CD4+ T cells and makes functional changes, eventually causing chronic spinal cord inflammation [8].
Patients with HAM/TSP have motor, sensory, and autonomic disturbances, which may affect gait performance, balance maintenance, and functional movement. Studies show that the first clinical presentations of HAM/TSP are muscle weakness and spasticity in the lower extremities. Muscle weakness occurs mostly in the hip flexors and ankle dorsiflexors, and spasticity frequently obvious in hip adductors and ankle plantiflexes [9]. Clinical features are mainly compatible with cord pathology, nevertheless imaging and autopsy studies also show inflammation in the brain. Patients with HAM/TSP illustration spastic paraparesis and sphincter disturbance, as well as sensory disturbances of the lower limbs.
The diagnosis of HAM/TSP was proved by positive ELISA and western-blot (WB) tests in both blood and cerebrospinal fluid (CSF) [6]. After the first report of TSP/HAM in 1985 and the explanation of WHO’s diagnostic criteria in 1988, recently the new criteria of HAM/TSP was described. The Osame criterion contemplates 2 states definite and probable. When the patient has both slowly progressive paraparesis and anti-HTLV-I antibodies in the blood and CSF, known as “definite”, whereas the “probable” level contains progressive myelopathy in patients with anti-HTLV-I antibodies either in the blood or CSF, but not in both, or myelopathy that is not steady with the above explanation in the presence of anti-HTLV-I antibodies in blood and CSF. In another criterion which is called by Castro-Costa et al. [2]. also there are 2 states same as the Osame criterion.
The classification of the “definite” diagnosis contains progressive spastic paraparesis that might be accompanying by sensory and sphincter signs or symptoms, the existence of anti- HTLV-I antibodies in the serum and CSF, proved by WB and/or positive PCR for HTLV-I in the blood and/or CSF. The diagnosis of HAM/TSP is “probable” if demonstrates monosymptomatic signs hyperreflexia or lower extremity spasticity or upward plantar reflex, sensory changes and anti-HTLV-I antibodies in serum and CSF proved by WB and/or positive PCR for HTLV-I in serum and/or CSF [10]. one of the useful diagnostic factors of HAM/TSP is high HTLV-1 CSF PBMC ratio. Treatment of TSP/ HAM remnants disappointing and symptomatic treatment is the mainstay of therapy [5].
Case report
A 53- year old woman who had right hemiparesthesia with inability to walkway admitted to the hospital in 2002. At first admission, because her brain and spinal MRI was found to be completely normal, no diagnosis had been done During the course, the patient’s symptom had become more severe and was slowly progressed until she had suffered from paraparesis. Then she was diagnosed as spinal stenosis and underwent the lumbar operation. However, no improvement was occurred after the surgery.
Meanwhile her sister developed the right-side hemiparesis as well and after careful work-up, she was diagnosed as MS. Therefore, for the second time, the neuroimaging work-up was done and brain and cervical MRI revealed the dissemination of hypersignal areas consistent with demyelinating lesions in the brain and cervical cord (Figure 1a, 1b). The DMT (Disease Modifying Treatment) was initiated for her, but she did not respond. In 2016, she had been referred to us. On the first visit she was wheelchair bound due to paraplegia. In neurological exam, the cranial nerves examination was normal. On the motor examination, the strength in the upper limbs were 5/5 with DTR+3 but the lower limbs were 0/5. On the sensory examination, she has sensory deficit below the T12 level, and the proprioceptive and position senses were abnormal in the lower limbs as well. She had an extensor plantar response (Babinski sign was positive). The DTR=+3 and the cerebellar examination (finger to nose and heel to shin) was normal.
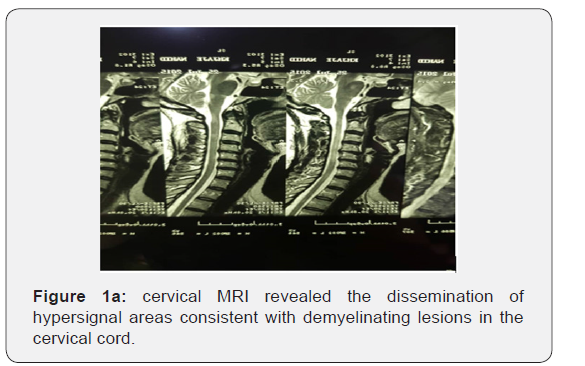

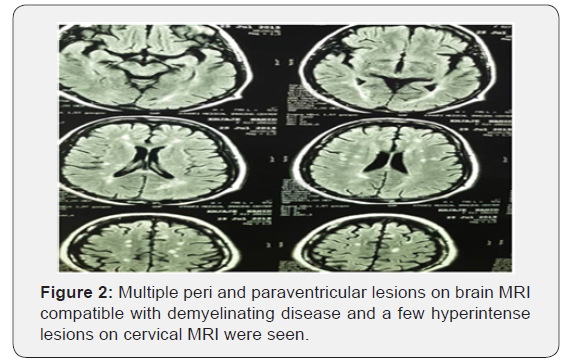
Multiple peri and paraventricular lesions on brain MRI compatible with demyelinating disease and a few hyperintense lesions on cervical MRI were seen. She did not have any enhanced lesions as well (Figures 1 & 2). The Visual Evoked Potentials (VEPs), were found to be pathologic bilaterally consistent with the damage of both optic nerves. (125s /137 s). In the laboratory data, JC virus and BK virus DNA was negative but anti HTLV1 Ab in both CSF and blood were positive. The diagnosis of HAM/ TSP was definite with PCR in the serum that 66540 copies/ml of HTLV-1 RNA was detected. The HTLV1 antibody in the serum and CSF was also positive. This patient was evaluated according to Osame & Castro-casta et al. [2]. criteria for HAM/TSP diagnosis. The Osame criterion contemplated both slowly progressive paraparesis and anti HTLV-1 antibodies in blood and CSF as definite. Therefore, according to this criterion the diagnosis of the HAM/TSP becomes apparent. The classification of the definite diagnosis by Castro-casta et al. [2]. contains progressive spastic paraparesis and the presence of anti HTLV-1 antibodies in blood and CSF proved by positive PCR for HTLV-1 in blood and/ or CSF. According to this theory the presence of the HAM/TSP is proven. The patient had no history of blood transfusion. Her sister who is known case of multiple sclerosis also was evaluated but she did not show seropositivity for HTLV-1,2. Cerebrospinal fluid investigation of our patient revealed the presence of, the OCB and high IgG index. Finally, the patient was diagnosed as HAM/TSP superimposed on Multiple Sclerosis.
Discussion
According to the patient history, she has had a progressive paraparesis with sensory and urinary dysfunction. The brain and cervical MRI was found to be compatible with MS according to Mc Donald criteria. In addition, her sister has been diagnosed with MS. Although she was diagnosed as MS, because the clinical finding was mainly consistent with myelopathy presenting with progressive spastic paraplegia, sensory level and sphincter dysfunction, we were suspicious about the other causes of progressive spastic paraplegia such as HTLV1 infection. The HTLV1 IgG antibody test in the serum was found to be positive and the HTLV1-2 DNA of virus was assessed in the serum and it was positive, too.
We were thinking the simultaneous presence of MS and HTLV1 infection in our patient. For justification, LP was done and interestingly again, the OCB was positive, and IgG index was also high. The HTLV1 DNA by PCR also was positive in the CSF as well. Therefore, HTLV1 myelopathy, HAM was ascertained finally.
In our opinion, if we encounter with a progressive spastic paraplegia and sphincter dysfunction as well as sensory problems, it is highly recommended to check the level of HTLV 1-2 antibody in the serum by WB or PCR methods especially when we are working in endemic area with high HTLV infection.
Result
1. As far as the above case has been demonstrated in terms of approach to a patient with familial history of HTLV1, we have to be concern to check the HTLV1 antibody in the blood by WB or PCR toll. If the blood test was positive, for certifying of HAM/TSP diagnosis, then assessment of CSF could be the second step.
2. In endemic area of the world with high prevalence of HTLV infection, in all MS patients who are a progressive course of myelopathy, we have to check the HTLV1 antibody in the blood by WB or PCR toll. If the blood test was positive, for certifying of HAM/TSP diagnosis, then assessment of CSF could be the second step.
3. The brain and cervical MRI, is not a sensitive investigation method for differentiation or rule out of HTLV1 and MS. Therefore, if we are suspicious to HTLV1 diagnosis, it is suitable to check the HTLV1 antibody in the blood by WB or PCR toll. If the blood test was positive, for certifying of HAM/TSP diagnosis, then assessment of CSF could be the second step.
4. We suppose that the PML viral load could be a prognostic value in HAP/TSP. Therefore, if the viral load was higher, the prognosis of myelopathy could be worsened.
References
- Liu H, Zhang JN, Zhu T (2012) Microsurgical treatment of spinal epidermoid and dermoid cysts in the lumbosacral region. J Clin Neurosci 19(5): 712-717.
- Manara R, Severino M, Mandari R, Mattisi G, Dal Pozzo S, et al. (2008) Chronic cystic lesion of the sacrum: characterisation with diffusionweighted MR imaging. Radiol Med 113(5): 739-746.
- van Aalst J, Hoekstra F, Beuls EA, Cornips EM, Weber JW, et al. (2009) Intraspinal dermoid and epidermoid tumors: Report of 18 cases and reappraisal of the literature. Pediatr Neurosurg 45(4): 281-290.
- Hamby WB (1944) Tumors in the spinal canal in childhood. II. Analysis of the literature of a subsequent decade (1933-1942); report of a case of meningitis due to an intramedullary epidermoid communicating with a dermal sinus. Journal of Neuropathology & Experimental Neurology 3(4): 397-412.
- French LA, Peyton WT (1942) Mixed tumors of the spinal canal. Arch Neur Psych 47(5): 737-751.
- Manno NJ, Uihlein A, Kernohan JW, (1962) Intraspinal epidermoids. J Neurosurg 19: 754-765.
- Matera RF, Martino A, (1945) Epidermoide gigaate infectado de cola de caballo. Arch Neurochir 2: 87-96.
- Ogden AT, Khandji AG, McCormick PC, Kaiser MG (2007) Intramedullary inclusion cysts of the cervicothoracic junction. Report of two cases in adults and review of the literature. J Neurosurg Spine 7(2): 236-242.
- Feldenzer JA, Mc Gauley JL, McGillicuddy JE (1989) Sacral and presacral tumors: Problems in diagnosis and management. Neurosurgery 25(6): 884-891.






























