Should Fatigue Be Considered as A Long-Term Sequela After Sepsis?
Gundula Seidel1,2*, Theresa Götz3 and Farsin Hamzei1,2
1Department of Neurology, Moritz Klinik Bad Klosterlausnitz, Germany
2Department of Neurology, Section of Neurological Rehabilitation, Jena University Hospital, Germany
3Department of Neurology, Section of Neurological Rehabilitation, Jena University Hospital, Germany
Submission: December 04, 2018; Published: March 13, 2019
*Corresponding author: Gundula Seidel, Moritz Klinik, Herrmann-Sachse-Str 46, 07639 Bad Klosterlausnitz, Germany
How to cite this article: Gundula S, Theresa G, Farsin H. Should Fatigue Be Considered as A Long-Term Sequela After Sepsis?. Open Access J Neurol Neurosurg. 2019; 10(1): 555778. DOI: 10.19080/OAJNN.2019.10.555778.
Abstract
Objective: Cognitive long-term impairment is a relevant sequela after sepsis. Certain diseases accompanied with cognitive decline are often also associated with fatigue (e.g. multiple sclerosis). The aim of this pilot report is to investigate if sepsis survivors with persistent cognitive deficits also suffer from fatigue syndrome.
Methods: In this cross-sectional study, 24 survivors of severe sepsis with persistent cognitive deficits were being examined by means of a neuropsychological test battery and interviewed on the basis of two German fatigue scales.
Result: 22 of the 24 sepsis survivors (91.7%) reported relevant fatigue symptoms. Considering their most relevant impairment of cognitive domain (alertness), sepsis survivors showed a close relationship between alertness and fatigue (higher fatigue scores were associated with lower alertness performance).
Conclusion: Fatigue is also a long-term sequela of sepsis survivors with persistent cognitive decline. As a result, this should be considered in rehabilitation of sepsis survivors, e.g. by a specific fatigue treatment and / or a training of alertness. In addition, this also raises the question whether sepsis survivors without cognitive impairment also suffer from fatigue syndromes, which could be another promising field of research.
Keywords: Sepsis, Fatigue, Cognitive outcome, Rehabilitation
Abbrevations: WEIMuS: Würzburger Erschöpfungsinventar; FSMK: Fatigue Skala für Motorik and Kognition; ICU: Intensive Care Unit
Introduction
Sepsis survivors have an increased risk for a long-term cognitive impairment which could affect various key domains of neuropsychological functioning, e.g. attention, memory or executive functioning [1,2]. Cognitive decline has been also detected in connection with certain diseases associated with fatigue (e.g. multiple sclerosis or stroke). In this regard, fatigue is defined as an overwhelming sense of tiredness, lack of energy or feeling of exhaustion [3]. A systematical investigation if fatigue is also a relevant long-term outcome in sepsis survivors suffering from persistent cognitive deficits has not been conducted so far. Therefore, the aim of this pilot observational study was to analyze if sepsis survivors with cognitive impairment also suffer from fatigue.
Participants and Methods
The inclusion criteria were a sepsis or septic shock episode with subsequent cognitive impairment dating back to at least more than one year. Sepsis and septic shock were defined according to the sepsis-3 consensus [4]. To verify cognitive impairment, participants were tested with a comprehensive neuropsychological battery targeting different domains of cognition: alertness, divided attention, selective attention, working memory, memory span, learning capacity, delayed recall and rate of decay, visuo-spatial ability, and executive function. Participants were defined as cognitively impaired if they reached a performance below one standard deviation at least in two cognitive domains a or if they had a performance below 1.5 standard deviation at least in one domain.
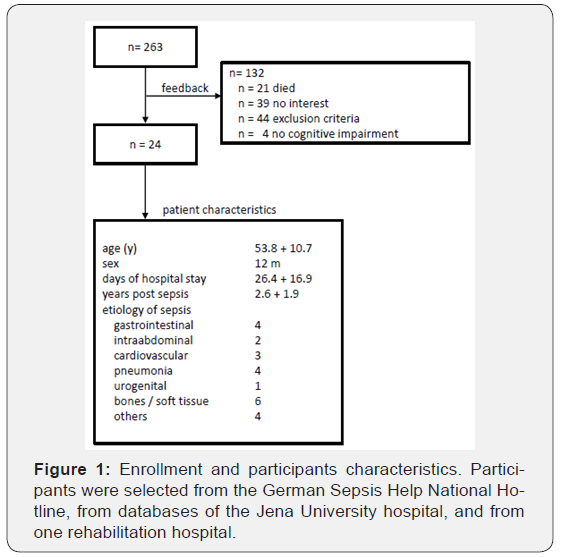
Exclusion criteria were history of other neurological diseases (e.g. brain trauma, stroke, parkinson or multiple sclerosis) which alter the brain. Psychiatric symptoms (e. g. depression or schizophrenia). Further distinctions with regard to participants selection and characteristics are listed in Figure 1. 24 participants whose sepsis or septic shock with subsequent persisting cognitive impairment dates back to more than one year were included (12 male, age 53.8 ± 10.6, years post sepsis 2.6 ± 1.9). All participants gave their written informed consent before participating in the study. This study was approved by the local ethics committee.
For fatigue assessment, two German fatigue questionnaires were used (“Würzburger Erschöpfungsinventar” WEIMuS [5] and “Fatigue Skala für Motorik und Kognition” FSMK [6], both contain items for cognitive and motor fatigue symptoms and corresponding cut-off values. Analyzing the relation between cognitive impairment and fatigue, first for both WEI MuS and FSMK, the scores were transformed into z-values and separately averaged for Fatigue total, Fatigue cognitive and Fatigue motor. The averaged z-values for fatigue were correlated with the T-values of those cognitive domains that were affected in more than 50% of the included participants. The significance threshold was set at p ≤ 0.05 (one-tailed, corrected for multiple comparisons according to Bonferroni-Holm).
Results
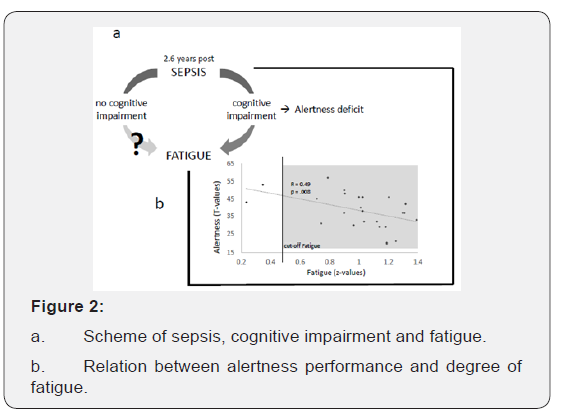
Tonic alertness was most frequently affected (in 13 participants; 54%), followed by working memory (in 11 participants; 46%), Memory functions were impaired in eight participants (33%) regarding their learning capacity; seven participants (30%) showed deficits in delayed recall, six participants (25%) in memory decay rate, and two participants (8%) in memory span. A total of 22 out of 24 participants (91.7%) reached Fatigue total scores in WEIMuS or FSMK which exceeded the cut-off. For WEIMuS, the mean value of Fatigue total score came to 43.0 (±176.2 SD), the result for the sub score Fatigue cognitive was 21.7 (±10.0 SD) and the sub score Fatigue motor amounted to 21.4 (±7.1 SD). For FSMK, the mean value of Fatigue total score came to 74.8 (±15.1 SD), the sub score Fatigue cognitive came to 36.5 (±9.0 SD) and the sub score Fatigue motor came to 38.3 (±6.9 SD). Thus, fatigue (i.e. the averaged z-values of Fatigue total of WEIMuS and FSMK) significantly correlates with tonic alertness (higher alertness deficit correlated with more fatigue; (Figure 2a).
Discussion
Our new approach is to describe fatigue as a long-term sequela in our study population of sepsis survivors with cognitive deficits. In this respect, we found a close association between fatigue and alertness deficits.
Alertness is supposed to be the most basic aspect of attention providing higher attention functions as well as higher cognitive demands [7]. With a deficit in this domain, the basic attention functions are in turn being reduced, which consequently affects higher cognitive processes. Thus, with regard to cognitive impairment sepsis survivors are forced to deploy considerably more cognitive effort in order to solve complex tasks. Therefore, sepsis survivors reported symptoms of fatigue. This suggestion links the manifestation of fatigue to the alertness deficit. On the other hand, sepsis is a systemic inflammatory process that develops fatigue similar to other inflammatory diseases (multiple sclerosis, rheumatism) [8]. Such a depiction would link an inflammation process to a development of fatigue.
In our report we did neither consider clinical parameters during the ICU stay (e.g. delirium) [2] nor psychological (e. g. depression and anxiety) and personality variable (e. g. resilience). The lack of this data thus requires further longitudinal studies to verify factors that encourage fatigue after sepsis (Figure 2b). Furthermore, we cannot give a general assessment about the prevalence of fatigue in sepsis survivors because we only investigated sepsis survivors with cognitive impairment.
Conclusion
Fatigue must be considered in sepsis survivors. To refine the intervention, specific treatments like cognitive behavioral therapy or graded exercise therapy should be taken into account during rehabilitation. Moreover, targeted training of alertness may also improve fatigue symptoms and should be considered as a constituent part in rehabilitation of sepsis participants.
Acknowledgement
The authors would like to thank all the participants who have made the study possible through their support. The authors would like to thank Sebastian Bähr for proofreading and linguistic correction of the manuscript.
Conflict of Interest
The authors declare no competing financial interests.
Funding
The study was supported by the German Center for Sepsis Control & Care (CSCC), funded by the Ministry of Education and Research (BMBF), grant no. 01 E0 1002.
References
- Cecil, Russell L, Smith LH (1985) Cecil textbook of medicine. WB Saunders.
- Clark DL, Boutros NN, Mendez MF (2011) The brain and behavior: an introduction to behavioral neuroanatomy. Cambridge, MA: Cambridge University, Press, UK.
- Liotta A, Advis JP, Krause JE, McKelvy JF, Krieger DT (1984) Demonstration of In vivo Synthesis of Pro-opiomelanocortin beta endorphin and alpha melanotropin-like species in the adult rat brain. J Neurosci 4(4): 956-965.
- Chrousos GP (1995) The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332(20): 1351-1362.
- McPartland JM, Guy GW, Di Marzo V (2014) Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PLoS ONE 9(3): e89566.
- Capobianco JD (2005) The Collected Writings of Robert G, Thorpe DO, FAAO. Indianapolis, In: American Academy of Osteopathy, USA.
- Mcpartland JM (2008) The Endocannabinoid System: An Osteopathic Perspective. The Journal of the American Osteopathic Association. 108(10): 586-600.
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D (2000) Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation 102(11): 1239-1244.
- Catherine Monk, Pavel Kovelenko, Lauren M Ellman, Richard P Sloan, Emilia Bagiella, et al.(2001) Enhanced stress reactivity in paediatric anxiety disorders: implications for future cardiovascular health. International Journal of Neuropsychopharmacology 4(2): 199-206.
- Yeragani VK, Rao KR, Pohl R, Jampala VC, Balon R (2001) Heart rate and QT variability in children with anxiety disorders: A preliminary report. Depression and Anxiety13(2): 72-77.
- Thayer JF. Stop That! Inhibition sensitization and their neurovascular concomitants. Scandinavian Journal of Psychology (43): 123-130.
- Thayer JF (2009) Vagal tone and the inflammatory reflex. Cleve Clin J Med 76(Suppl_2): s23: 26.






























