Facial Weakness in Heterozygous Carriers of Calpain3 mutation
Ruben Attali1, NasimWarwar2, Harel Shein2, Irina Gurt2, Judith Melki PhD3, Yakov Fellig4, Zohar Argov MD5, Stella Mitrani-Rosenbaum1 and Marc Gotkine5*
1Goldyne Savad Institute of Gene Therapy, Hadassah Hebrew University Medical Center, Israel
2Monique and Jacques Roboh Research Laboratory and Altura Department of Human Genetics, Hadassah Hebrew University Medical Center, Israel
3Unite Mixte de recherche (UMR)-1169, Inserm and University Paris Saclay, France
4Department of Pathology, Hadassah Hebrew University Medical Center, Israel
5Agnes Ginges, Department of Neurology, Hadassah Hebrew University Medical Center, Israel
Submission: December 04, 2018; Published: April 02, 2018
*Corresponding author: Marc Gotkine, Hadassah Medical Center, Agnes Ginges Department of Neurology, POB 12000, Jerusalem 91120, Israel,Tel: 972-2-6776941; Fax: +97226437782, Email: marc@gotkine.com
How to cite this article: Ruben A, NasimWarwar, Harel S, Irina G, Judith M P. Facial Weakness in Heterozygous Carriers of Calpain3 mutation. Open Access J Neurol Neurosurg. 2018; 7(3): 555714. DOI: 10.19080/OAJNN.2018.07.555714
Abstract
Background: The limb-girdle muscular dystrophies (LGMD) are a heterogeneous group of disorders characterized by weakness and wasting of the pelvic and shoulder girdle muscles. Various clinical features may allow suspicion of a particular molecular diagnosis, including: age of onset, relative muscle involvement, cardio-respiratory involvement, presence of contractures and inheritance type. LGMD2A is due to mutation in the CAPN3 gene, resulting in a deficiency of the enzyme calpain.
Objective: Describe a family found to have a calpain mutation, in which heterozygous carriers manifested symptomatic facial weakness.
Methods: We describe a woman from a Jewish, Persian, consanguineous family who presented with an atypical myopathy pattern, including prominent facial muscle weakness. Clinical and genetic analysis was performed on the index patient and family members.
Results: Symptomatic facial weakness was found in her mother and maternal aunts. Exome sequencing identified a homozygous mutation in the CAPN3 gene, confirming recessively inherited LGMD2A. The mother and symptomatic aunts were found to be heterozygous for the mutation.
Conclusion: The presence of symptomatic facial weakness in heterozygous carriers of this CAPN3 mutation suggests either manifesting carrier status, or involvement of an independent genetic cause.
Keywords: LGMD; CAPN3; Calpain; Muscular dystrophy; Manifesting carrier
Introduction
Limb girdle muscular dystrophies (LGMD) are a group of hereditary myopathies that affect primarily the proximal limb musculature, but usually spare the facial muscles [1-3]. LGMD2A is a recessive disorder due to calpain deficiency resulting from homozygous or compound heterozygous mutations in the CAPN3 gene [4,5].The phenotype is highly variable, and may include —early scapular winging and rarely, facial weakness, thus mistakenly suggesting the diagnosis of facio scapula humeral dystrophy (FSHD)[6,7]. Here we describe an atypical pedigree with calpain deficiency, where both the homozygous index patient, and her heterozygous mother and aunts, had prominent facial weakness.
Pedigree
The index patient (IV-2) presented with waddling gait from early childhood. Her parents (III-6 and III-7) were first cousins, from Jewish Persian origin (). At age 18 she presented to our neuromuscular clinic with gait deterioration and difficulty climbing stairs. Examination revealed prominent facial muscle weakness (Figure 2-left panel), scapular winging and proximal limb weakness, most pronounced in the lower limbs. Gait was waddling and there was a positive Gower's sign. There were no contractures, calf hypertrophy nor evidence of cardiorespiratory involvement. CPK was elevated in the 650-1200 range and EMG was interpreted as myopathic.
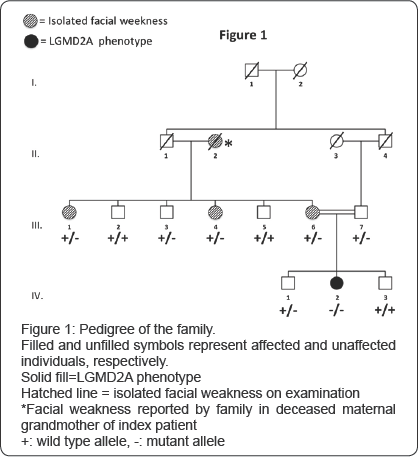
The mother (III-6] complained of a long histoiy of facial weakness, also present in two of her sisters (III-l and III-4], which the family had always referred to as a "bulldog face” (Figure 2-right panels]. They also reported that their own mother-the index patient’s maternal grandmother (II-2, deceased]-had the same distinctive facial features. Examination of the mother and the two affected aunts revealed isolated facial weakness (Figure 2-right panels]. CPK was normal in the mother and affected aunts. The father (III-7] and maternal uncles (III-2, III-3 and III-5] were not affected. Microscopic examination of quadriceps muscle biopsy submitted from the index patient was consistent with a non-specific muscular dystrophy. Immuno histochemical stains for sarcoglycans, dysferlin and merosin were normal.

Genetic analysis
Informed consent was obtained for all family members undergoing genetic analysis. The length of both D4Z4 alleles was normal, excluding FSHD1. Because the inheritance pattern was unclear, genome wide linkage was performed, using 250KSNP microarray (Affymetrix), and analyzed under various inheritance models (X-linked, autosomal recessive and autosomal dominant). The LOD scores obtained excluded an X-linked model. One region at 20q11-13 (with a LOD score Zmax=3/0045 at □=0.0) was retained for an autosomal recessive inheritance model, with a total of 240 candidate genes, and 2 further loci at 6q21 and 18q23 (with a maximum LOD score Zmax=2.4 at  =0.0]were retained for an autosomal dominant inheritance model, with 18 and 22 candidate genes respectively. The large number of genes in each of the candidate regionsled us to perform whole exome sequencing of the index patient.
=0.0]were retained for an autosomal dominant inheritance model, with 18 and 22 candidate genes respectively. The large number of genes in each of the candidate regionsled us to perform whole exome sequencing of the index patient.
Exome sequencing
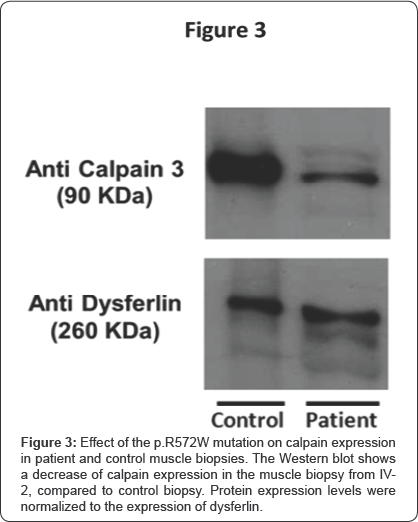
Exome sequencing (BGI Shenzhen) reached a mean sequencing depth of 126 fold with coverage of 98.76% (with 90.78% of the targets covered ≥20x). Mutations in FSHD2 and SMCHD1 were excluded (covered at 99.83% with a mean depth of 111x). The exome sequencing failed to identify segregating mutations among the candidates listed by the previous linkage analysis.In contrast, when crossed with the 333 genes associated with neuromuscular diseases (http://www. musclegenetable.fr/) the gene list of all the variants obtained by exome sequencing identified homozygote missense mutations in 4 different genes involved in neuromuscular diseases. All 4 were Sanger sequenced, but only the homozygote mutation in CAPN3, identified in the index patient, segregated within the pedigree. The mutation is a homozygous C to T nucleotide substitution at position 1714 (NM_000070.2) leading to an amino acid change p.R572W.Western blot analysis revealed a low level of Capn3 protein in the muscle of the index patient (Figure 3). The heterozygous mutation was confirmed by Sanger sequencing to be present in the father (III-7) and uncle (III-3)- who were completely asymptomatic-and the mother (III-6) and two maternal aunts (III-1 and III-4), who all had isolated facial weakness.
Discussion
The p.R572Wcalpain mutation has been described in several patients suffering from LGMD2A. The majority of cases have been compound heterozygotes, though a single homozygous patient with a LGMD pattern, sparing the facial muscles, has been reported [8]. Facial weakness is an established manifestation of calpain deficiency [6,7], which seems to be unrelated to the specific mutation, and variably present in patients with identical mutations [9,10]. Thus the facial weakness, displayed by the index patient described here is consistent withcalpainopathy. The mother and the maternal aunts were heterozygous for the mutation, and all of them had symptomatic facial weakness, suggesting manifesting carrier status. The phenomenon of manifesting carrier status is well established in X-linked myopathies such as in dystrophinopathy and X-linked myotubular myopathy, where skewed X inactivation occurs [11,12]. Symptomatic carriers of autosomal recessive myopathies are rare, and have never been described in calpainopathy, but have been described in heterozygous carriers of mutations in fukutin [13] and dysferlin [14].
The manifesting carrier hypothesis is attractive, given the prominent facial weakness of the index patient, but does not address the male members of the family, who were also heterozygous for the mutation, yet asymptomatic. The manifestation of facial weakness in only the female carriers could be related to sex-limited inheritance, but linkage analysis with the relevant parameters, excluded this possibility since the LOD score at the locus spanning the CAPN3 gene on chromosome 15 was negative. This raises alternative possibilities: that additional genetic factors are acting as modifiers, or that the facial weakness could be due to a separate genetic abnormality. This is the first report of facial weakness in heterozygous carriers of a CAPN3 mutation. Understanding the mechanisms determining phenotype appearance in carriers could shed light on the relationship between genotype and phenotype in LGMD2A.
References
- Bushby K (2009) Diagnosis and management of the limb girdle muscular dystrophies. Practical neurology 9(6): 314-323.
- Lo HP, Cooper ST, Evesson FJ, Seto JT, Chiotis M, et al. (2008) Limb- girdle muscular dystrophy: Diagnostic evaluation, frequency and clues to pathogenesis. Neuromuscular Disord 18 (1): 34-44.
- Nigro V, Aurino S, Piluso G (2011) Limb girdle muscular dystrophies: update on genetic diagnosis and therapeutic approaches. Current opinion in neurology 24 (5): 429-436.
- Sorimachi H, Toyama-Sorimachi N, Saido TC, Kawasaki H, Sugita H, et al. (1993) Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. The Journal of biological chemistry 268 (14): 10593-10605.
- Richard I, Roudaut C, Saenz A, Pogue R, Grimbergen JE, et al. (1999) Calpainopathy-a survey of mutations and polymorphisms. Am J Hum Genet 64(6):1524-1540.
- Leidenroth A, Sorte HS, Gilfillan G, Ehrlich M, Lyle R, Hewitt JE (2012) Diagnosis by sequencing: correction of misdiagnosis from FSHD2 to LGMD2A by whole-exome analysis. Eur J Hum Genet 20 (9): 999-1003.
- Sacconi S, Camano P, de Greef JC, Lemmers RJLF, Salviati L, et al. (2011) Patients with a phenotype consistent with facioscapulohumeral muscular dystrophy display genetic and epigenetic heterogeneity. Journal of Medical Genetics 49(1): 41-46.
- Richard I, Brenguier L, Dincer P, Roudaut C, Bady B, et al. (1997) Multiple independent molecular etiology for limb-girdle muscular dystrophy type 2A patients from various geographical origins. American journal of human genetics 60(5): 1128-1138.
- Piluso G (2005) Extensive scanning of the calpain-3 gene broadens the spectrum of LGMD2A phenotypes. Journal of Medical Genetics 42(9): 686-693.
- Blazquez L, Azpitarte M, Saenz A, Goicoechea M, Otaegui D, et al. (2008) Characterization of novel CAPN3 isoforms in white blood cells: an alternative approach for limb-girdle muscular dystrophy 2A diagnosis. Neurogenetics 9(3): 173-182.
- Tanner SM, Orstavik KH, Kristiansen M, Lev D, Lerman-Sagie T, Sadeh M, et al. (1999) Skewed X-inactivation in a manifesting carrier of X-linked myotubular myopathy and in her non-manifesting carrier mother. Hum Genet 104(3): 249-253.
- Yoshioka M, Yorifuji T, Mituyoshi I (1998) Skewed X inactivation in manifesting carriers of Duchenne muscular dystrophy. Clin Genet 53(2): 102-107.
- Schottlaender LV, Petzold A, Wood N, Houlden H (2015) Diagnostic clues and manifesting carriers in fukutin-related protein (FKRP) limb- girdle muscular dystrophy. Journal of the neurological sciences 348 (1-2): 266-268.
- Illa I, De Luna N, Dominguez-Perles R, Rojas-Garcia R, Paradas C, et al. (2007) Symptomatic dysferlin gene mutation carriers: characterization of two cases. Neurology 68(16): 1284-1289.






























