The Electrical Disinfection of the Skin in Presence of Foreign Bodies
Reynders Frederix P1*, Herteleer M2, Verhaegen J3, Verbeeck M4, Scheveneels K4and Reynders-Frederix C5
1Department of Orthopedic Surgery, University Hospitals Brussels, Belgium
2Department of Traumatology, Catholic University Leuven, Belgium
3Department of Microbiology, Catholic University Leuven, Belgium
4Bio-technology lab, Catholic University Leuven, Belgium
5Department of Fysiotherapy, University Hospitals Brussels, Belgium
Submission: July 05, 2017; Published: July 26, 2017
*Corresponding author: Reynders Frederix P, Department of Orthopedic Surgery University Hospitals Brussels, Campus Brugmann, Leuven, Belgium, Tel: 32473361420, Email: reynders52@hotmail.com
How to cite this article: Reynders F P, Herteleer M, Verhaegen J, Verbeeck M, Scheveneels K, Reynders-F C. The Electrical Disinfection of the Skin in Presence of Foreign Bodies. Nov Tech Arthritis Bone Res. 2017; 1(4) : 555567 DOI: 10.19080/NTAB.2017.01.555567
Abstract
Percutaneous pins are prone for infection, which can cause devastating effects on the bone and the surrounding tissues [1]. In the infected tissues, the bacterial adhesion causes the formation of a bio film. These bacterial bio films are made up of extracellular polymeric substances. They reduce the effectiveness of antibiotics. About 500 to 5,000 times the levels of antibiotic concentration than usual are needed in conditions with these bio films [2]. Several studies have reported a destructive effect of low amperage direct current on bacterial bio films and on the bacteria itself [3]. The low amperage direct current acts by two ways on the bacterial bio film and the bacteria. The first mechanism is the detachment of the bacteria within the bio films from the stainless steel and the second is by a direct bactericidal effect [4-7].
These findings provide thoughts for a new method of treatment for infections. Some studies show a bactericidal effect when the low amperage current is applied directly to the percutaneous pin or screw [8,9]. However if direct current is applied to the bone or the wound, it influences the healing process of these tissues [10]. Other studies have also shown a bactericidal effect when the electric current is applied to a surface such as an agar plate or the skin [11,12]. In clinical practice, these techniques would simplify the disinfection of the metal-skin interface.
We set up an experiment to corroborate the findings of the previous authors.
Materials and Methods Microorganisms
Staphy lococcus aureus (ATCC 25923) and Pseudomonas aeruginosa (ATCC 27853).
Electric current and electrodes
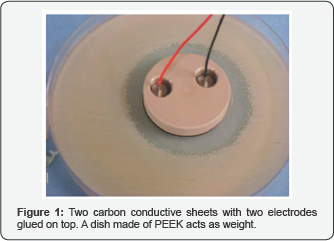
We used two semicircular conductive carbon electrodes (Getelec, Conductive Carbon BL 10000, Rue Condorcet 75, 92140 CLAMART, France) with a diameter of 48 mm and a thickness of 0, 5mm. Two small stainless steel electrodes were glued (RTV 1030 Flexible Silicone Adhesive) to the conductive carbon electrodes to transmit the current Figure 1.
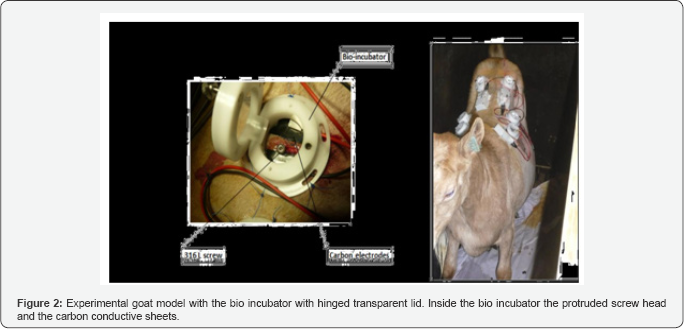
The direct current was provided by an adjustable current source (Temma 72‐6694, current calibrator, CPC Armley Road 150 leeds LS 12299) for the agar petri dish and a custom made constant current source for the goat model Figure 2. Both devices delivered no more than the specified total number of microamperes of direct current.
Experimental agar model
There were 3 different setups, each with two conductive carbon electrodes that were placed on a Mueller Hinton II Agar (Oxoid) that was incubated with S. Aureus, with a density of McFarland 0.5 and a 1cm gap between the electrodes Figure 3. We applied (4V, 1mA), (3, 5V; 500microA), (2, 3V, 82microA). An identical setup was created for a P. Aeruginosa incubated Mueller Hinton II agar plate.
In a fourth setup, an electromagnetic field was created by an electric current (Direct and alternating Current) through a metal wire which was not in contact with the agar medium. The influence of the electromagnetic field on the bacterial growth inhibition was examined.
Experimental animal model
We used a Saanen Goat for our experiments. Anesthesia The animal was given anesthetic induction using Xylazine (Vexylan 1ml/50kg) and Ketamine was given intravenously in doses of 1, 5ml/10kg during the procedure. The goat was intubated and Isoflurane was given in a 2% in 5 Liter Oxygen solvent during the procedure. Buprenorphine (Temgesic®) was given in doses of 1ml IM preoperatively and postoperatively to reduce the induction dose and post operative pain.
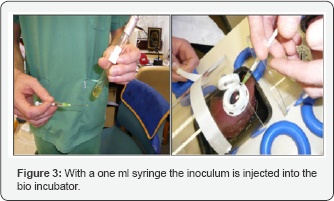
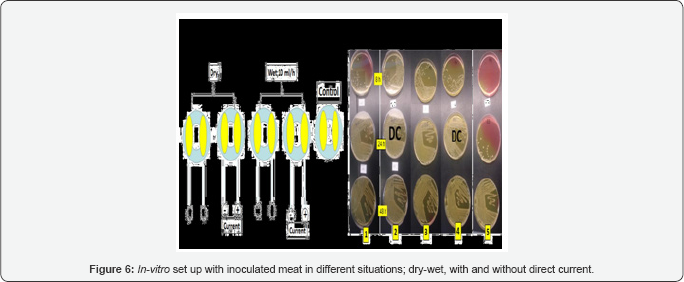
The goat was positioned in prone position on the operating table and its back was shaved with an electric shaver. Initially, we used to disinfect the concerned part of skin was with iodine 1% solution in alcohol. Since no growth was noticed, we decided not to disinfect the skin. Six stab incisions were made at 3 different levels on both sides of the spine. Percutaneous bone screws of surgical steel 316L were fixed on the transverse process of the lumbar vertebrae. Two carbon conductive pads were placed at a distance of the protruding screw head and covered underneath a cylindrical polyethylene bio-incubator with an hinged lid made of a transparent plastic membrane (Polymethyl methacrylate, Plexiglass®). All the wounds were inoculated with S. Aureus McFarland 0.5. This inoculation was done using a 1ml syringe Figure 3.
On three wounds, a 1, 4mA current was applied through the conductive carbon sheets for two weeks. A culture was taken from the stab wounds on every second day (Aptaca, Spa Brescia, Italy).
Experimental incubator model
A bio incubator model was set up in which we could control and measure the temperature, humidity (Escort Junior recorder EJ-HS-B-16, temp -20/60 °C, rh 0-100%) and current intensity (Fluke, 85 current meter) at all times. We used a transparent cylindrical incubator with a length of 80 cm and a diameter of 15 cm which was connected to a hot air blower Figure 4. Five pieces of fresh beef meat were placed in the bio incubators in the same way as was done in the experimental goat model. An adaptation was made to two bio-incubators by adding an infusion tube to the top of the bio-incubator. This enabled us to add saline (10ml/ hour) during the experiment. Comparison was made between the control (no DC and dry) and moisture electrodes with and without DC and two dry electrodes of which one conveyed DC.

Results
Experimental agar model

We noted a clear correlation between the current intensity and inhibition zone around both carbon pads. We also observed that ATCC 25923 S. Aureus generated a clearly larger zone than P. Aeruginosa (ATCC 27853) with the same intensity of current. Alternating current (100 A, 1.2 Volt with 15Hz) did not show any inhibitory effect on the bacterial growth Figure 5.
Also the electromagnetic field, created by an electric current (AC & DC) through a metal wire in offset position with the agar medium, did not inhibit bacterial growth.
Experimental goat model
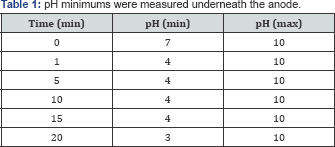
During the first twenty minutes of the experiment, we detected changes in pH, oxygen and chloride radicals. The radicals were measured between the pads the pH measured underneath the carbon pads. The pH minimums were measured underneath the anode and the pH maximums were measured underneath the cathode Table 1 & 2.
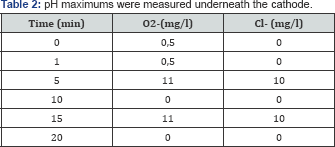
As a general outcome measurement, the non-appearance of bacterial growth was considered as success and the appearance as failure. Cultures were taken every two days. We noticed a tendency of early bactericidal effect using direct current in 3 out of 4 incubated screws. All the cultures, which were taken after one week were positive.
Experimental incubator model
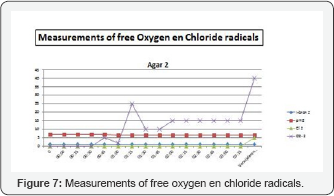
Cultures were taken after eight, twenty‐four and forty‐eight hours. All cultures were positive Figure 6 & 7.
Discussion
Electric current has proven to have a bactericidal effect in certain environments. Liu [12] described the effect of low amperage direct current in agar plates. He demonstrated the production of H2O2 at the cathode and chlorine at the anode. However, in his experiment he could not show an important reduction of bacterial growth activity around the anode. With the use of our conductive carbon electrodes, we could show an inhibition zone around the cathode and the anode. This difference might be due to the larger amperages we used. Liu used amperages of 10‐100microA in his studies while amperages as high as 82-1000microA were used in our studies. Another possible explanation is the difference in the quality of conductive materials used in the studies. The carbon conductive electrodes used in our studies might be better than the catheters Liu used.
Bolton [11] reported bactericidal activity on commensal skin flora of carbon‐containing positives electrodes that were placed on the intact skin of human subjects. Bolton used current density and intensity as different parameters in their experiments. He also stated that the bactericidal effect occurred only beneath the carbon-containing positive electrodes. In Bolton's opinion, the bactericidal effect was proportional to the current density and acidity under the patch. He also reported a reduced bactericidal effect in citrated sheep's blood.
Van der Borden [9] set up an experiment in which three pins were inserted into the lateral tibia of nine goats. Two of these pins were subjected to infection with a Staphylococcus epidermid is strain. 100microA direct current was applied directly to one pin. The cathode of the current source was connected to the pin and the anode was connected to the skin. After 21 days, the pins were taken out of the goats. It was found that infection developed in 89% of the control pins in contrast to a mere 11% of the direct current pins. They concluded that 100 microA direct currents were able to prevent clinical signs of infection around surgical stainless steel pin sites, even without the use of antibiotics in intentionally infected wounds.
Del Pozo [6] reported the use of low amperage direct current as a cure for infection in chronic foreign body osteomyelitis. They set up a rabbit model in which a stainless steel implant infected with Staphylococcus epidermidis was placed into the medullary cavity of the tibia. After four weeks they assigned the rabbits into 3 different treatment groups. A control group, a doxycycline treatment group and a 200microA treatment group. The treatment with electric current produced more bactericidal effect than the other two groups. They concluded that the treatment with electrical current was more effective, since it showed higher statistically significant bactericidal effect than the doxycycline therapy and control group (P=0.035)
Our study partially confirms the results of Liu and Bolton. We obtained an asymmetrical bactericidal effect underneath both carbon electrodes. More inhibition effect was seen under the positive electrode and to a lesser inhibition around the negative electrode. This difference might be due to the higher amperages we used or due to the carbon patch used in the study.
Our point of interest lies in the inhibition zones we demonstrated around the positive and negative carbon pads. This is due to the significant influences of low amperage direct current on bone healing. Kuzyk [10] reviewed the influences of direct electrical stimulation on fracture healing and concluded that the presence of a synovial pseudarthrosis, a large bone gap at the fracture site, or an osteomyelitis were absolute contraindications for direct electrical stimulation. Since these contraindications are often found in patients, we thought of a non-invasive electric current application to prevent infection.
There are many reasons for the bactericidal effect of low amperage current, in vitro. The most reasonable explanation is the production of toxic radicals as stated by many other authors [ 11]. However, in the case of Staphylococcus Aureus the production of oxygen radicals alone cannot explain this bactericidal effect. Staphylococcus Aureus is a catalase- positive micro-organism. The function of the catalase enzyme is to disintegrate hydrogen peroxide into water and oxygen. Another cause for the bactericidal effect is due to the strong acid-base environment. The electric current heavily influences the zone around the patch. Our measurements confirmed this hypothesis. These strong acid and base environments are hostile to Staphylococcus Aureus. That is the reason for creating a moisture environment in some of our bio-incubators. The humidity, together with the direct current, facilitates the electrolysis of water and the subsequent release of hydrogen ions at the positive electrode (ions are attracted to the electrode surface from the medium, providing electrons to the electrode) [11]. Another important aspect of our findings is the disrupting effect of electric current on the cell membrane. It is most likely that the effect is caused by a combination of all the three mechanisms.
Since these three systems are very complex and easily disturbed, they cannot function in an optimal way in any environment and are unlikely to sustain a bactericidal effect.
Conclusion
Percutaneous pin tract infections still remain an important problem. Wound care and antiseptic measures remain the gold standard in the prevention of pin tract infections. Low amperage direct current has proven to have bactericidal effects in different settings. Unfortunately we could not to corroborate these findings in our in vivo models. We postulate that, in vivo, more complex reactions occur at the skin-metal interface and they neutralize the effect we observed in vitro. Further research is warranted.
References
- Davies R, Holt N, Nayagam S (2005) The care of pin sites with external fixation. J Bone Joint Surg Br 87(5): 716-719.
- Nickel JC, Ruseska I, Wright JB, Costerton JW (1985) Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27(4): 61924.
- Valle A, Zanardini E, Abbruscato P, Argenzio P, Lustrato G, et al. (2007) Effects of low electric current (LEC) treatment on pure bacterial cultures. J Appl Microbiol 103(5): 1376-1385.
- Pareilleux A, Sicard N (1970) Lethal effects of electric current on Escherichia coli. Appl Microbiol 19(3): 421-424.
- van der Borden AJ, van der Werf H, van der Mei HC, Busscher HJ (2004) Electric-current-induced detachment of Staphylococcus epidermidis strains from surgical stainless steel. Appl Environ Microbiol 70(11): 6871-6874.
- del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R (2009) The electricidal effect: reduction of Staphylococcus and pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother 53(1): 41-45.
- Wellman N, Fortun SM, McLeod BR (1996) Bacterial bio films and the bioelectric effect. Antimicrob Agents Chemother 40(9): 2012-2014.
- Del Pozo JL, Rouse MS, Euba G, Kang CI, Mandrekar JN, et al. (2009) The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother 53(10): 4064-4068.
- van der Borden AJ, Maathuis PG, Engels E, Rakhorst G, van der Mei HC, et al. (2007) Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials 28(12): 2122-2126.
- Kuzyk PR, Schemitsch EH (2009) The science of electrical stimulation therapy for fracture healing. Indian J Orthop 43(2): 127-131.
- Bolton L, Foleno B, Means B, Petrucelli S (1980) Direct-current bactericidal effect on intact skin. Antimicrob Agents Chemother 18(1): 137-141.
- Liu WK, Brown MR, Elliott TS (1997) Elliott, Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother 39(6): 687-695.






























