Assessment of Pharmaceutical Equivalence and In-Vitro Bioequivalence of Four Brands of Commercially Available Nifedipine Tablets in Swaziland
Adefolarin A Amu1, Bheki Masondo1, Ayanda Lukhele1, Nokusho Dlamini1, Nhlanhla Nhleko1 and Julius O Soyinka1,2*
1Department of Pharmacy, Swaziland Christian University, Southern Africa
2Department of Pharmaceutical Chemistry, Obafemi Awolowo University, Nigeria
Submission: May 26, 2018; Published: August 14, 2018
*Corresponding author: Julius O Soyinka, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria, Tel: Email: +2348035822785 Email: juliussoyinka@gmail.com
How to cite this article: Adefolarin A A, Bheki M, Ayanda L, Nokusho D, Nhlanhla N, Julius O S. Assessment of Pharmaceutical Equivalence and In-Vitro Bioequivalence of Four Brands of Commercially Available Nifedipine Tablets in Swaziland. Mod Appl Bioequiv Availab. 2018; 4(1): 555626.
Abstract
Background: Nifedipine is key in the management of hypertension, but also represents additional financial burden, particularly with the use of branded products. The availability of generic products permits generic substitution with a much-reduced cost of treatment. However, only generic products that offer similar bioavailability with the innovator should be considered. This study was designed to assess the pharmaceutical equivalence and in-vitro bioequivalence of generic Nifedipine tablets within Swaziland.
Objectives: Assessment of quality and in-vitro bioequivalence of four brands of Nifedipine tablets available in Swaziland in order to ascertain their safety, efficacy, stability and pharmaceutical equivalence.
Methods: Four brands of immediate release Nifedipine 20 mg tablets, coded PM, PN, EA and EN respectively, were obtained from retail pharmacy outlets and the central medical store. They were assessed for quality using the United States Pharmacopoeia (USP) guidelines. Characteristics assessed for the determination of their pharmaceutical equivalence were physical appearance, weight variation, diameter, hardness, friability, drug content and disintegration, while the determination of their dissolution rate was used in the assessment of their in-vitro bioequivalence.
Results: Sample PM, did not comply with the official specifications for weight variation, whilst all the others complied. Both samples PM and PN complied with the specifications for diameter and the thickness variation but did not pass the test for hardness. However, both samples EA and EN complied with the specification for hardness. None of the four samples complied with the specifications for both the disintegration and the dissolution rate, but they all complied for the specifications of friability and drug content.
Conclusion: Results from the study have shown that none of the brands is of acceptable quality, and the brands are not pharmaceutically equivalent. Moreover, none of the brand is likely to produce acceptable oral bioavailability, which is deduced from in-vitro bioequivalence. Thus, switching or substituting brands of Nifedipine tablets for patients should be guided by a critical assessment of the dissolution rate and other quality parameters.
Keywords: Pharmaceutical Equivalence; In-vitro Bioequivalence; Nifedipine; Bioavailability
Abbreviations: WHO: World Health Organization; BCS: Biopharmaceutics Classification System
Introduction
Hypertension is a major public health problem world-wide with its attendant high morbidity and mortality. Almost a billion of the world’s adult population exhibited signs and symptoms of hypertension with rather low detection, control and treatment rates [1]. The disease is generally managed through medication use and dietary or lifestyle changes. Nifedipine [Dimethyl-2,6-methyl-4-)2-nitrophenyl)-1,4-dihydropyridine-3, 5-dicarboxylate] (Figure 1), is a calcium channel blocking agent of the dihydropyridine type which is commonly employed in the management of systemic hypertension and angina pectoris [2]. Nifedipine has a short elimination half-life of 2-4 hours, and it is rapidly and completely absorbed over the entire gastrointestinal tract, despite its low water solubility. It is mainly administered orally in a form of both immediate release and sustained release tablet formulations, and due to the lack of major metabolic adverse effects, it is a relatively safe and well tolerable medication [3].
Two or more drugs are said to be bioequivalent, when they are pharmaceutically or chemically equivalent products, and they produce comparable bioavailability characteristics in any individual when administered in equivalent dosage regimen [4]. Bioavailability is a term used in pharmacology to refer to the degree and rate at which an administered drug is absorbed by the body’s circulatory system, the systemic circulation. It determines therapeutic efficacy because it affects onset, intensity and duration of therapeutic response of a drug [5].
There is a growing concern about the availability of substandard pharmaceutical products to the general public in developing countries. Such products have therapeutic as well as social and economic implications. There is little data available which points to the reasons for products being substandard, but the majority of literature reports contain no concrete evidence and assume the products to be counterfeit [6,7]. There are, however, other reasons for products being substandard, such as poor quality control during manufacture or decomposition of the active ingredient(s) [8]. The quality of drugs in lessdeveloped settings is inadequate, although concrete evidence is largely not documented. Reasons for poor quality include the widespread counterfeiting of medicines, decomposition of the active ingredient in drugs due to high temperature and humidity of storage, and poor quality assurance during the manufacture of medicinal products [8].
In countries like Swaziland, where the drug control is quite inadequate, the quality of marketed drug products cannot be guaranteed. Evaluation of some of the marketed products could give an insight as to the quality of products sold & consumed and could lay basis for future corrective measures. If a drug, upon laboratory testing in accordance with the specifications it claims to comply with, fails to meet the specifications, then it is classified as a substandard drug. Substandard drugs are drug products that do not meet quality specifications set for them and they may either be genuine or counterfeit medicinal products [7]. The World Health Organization (WHO) defines a counterfeit medicine as “a medicine which is deliberately and fraudulently mislabelled with respect to identity and/or source [9]. Counterfeiting can apply to both branded and generic products and counterfeit products may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient active ingredients or with fake packaging [9]. There are several reports on the presence of medicines or drugs of doubtful quality (substandard medicines) on the market in both developed and developing countries [6,7]. Consequences of substandard medicines include: reduction in bioavailability as a result of reduction in dissolution of active ingredient. In general, the high-price associated with some branded products may predispose patients to opt for generic products.
In Swaziland, many generic products are in circulation, and they are often preferred by the populace because of the prevailing poor socioeconomic status. This trend has helped to curtail rising in pharmaceutical expenditure, especially in lowto middle-income countries [10]. However, generic substitution should not be based solely on the initial cost of treatment but on the overall cost effectiveness of pharmacological treatment [10]. As a result, a standard has been set for generic substitution. Interchangeability is permitted when the generic product demonstrates bioequivalence and therapeutic equivalence with the innovator.
Bioequivalence of a generic product could be determined by either in-vivo or in-vitro studies. In-vivo bioequivalence studies are frequently used to establish therapeutic equivalence, but this approach is usually expensive and more rigorous and may require clinical trial or study expertise [11]. In-vitro dissolution profiles are proxies for establishing bioequivalence when the drug meets the criteria prescribed for a Biopharmaceutics Classification System (BCS) biowaiver [12]. The BCS considers three major factors: dissolution, solubility and intestinal permeability, which influence the rate and extent of drug absorption from immediate release solid oral dosage forms [12]. Biopharmaceutics Classification System class II drugs exhibit low solubility and high permeability characteristics. Their oral absorption is mostly governed by in vivo dissolution; the solubility and the dissolution rate are therefore key determinants for the oral bioavailability of these drugs. This implies that a small increase in the dissolution rate will result in a multifold increase in bioavailability [13]. Dissolution testing is an important tool employed in pharmaceutical development and in quality evaluation of solid dosage formulations [13], especially poorly soluble drugs. Dissolution testing is also used as a surrogate for in-vivo drug release and bioavailability of drugs.
The pharmaceutical quality and dissolution properties of commercial Nifedipine tablet brands have been a major concern to researchers and healthcare professionals. As a result, several studies have been undertaken to assess the pharmaceutical and biopharmaceutical quality of Nifedipine tablet formulations available to the healthcare delivery system of various countries. The pharmaceutical quality evaluation of ten commercial brands of 20mg sustained release Nifedipine tablets in Nigeria showed that only four were pharmaceutically equivalent [14]. The dissolution properties of prolonged release Nifedipine tablets sampled on the Belgian market indicated that the brands were dissimilar and were therefore not interchangeable [15]. The dissolution of Nifedipine tablets produced in five different factories in China were found to comply with standards of the Chinese Pharmacopoeia but failed to meet the specifications of both the British Pharmacopoeia and the United States Pharmacopoeia [16]. Developing countries will benefit from generic products, unfortunately the resources for testing drug quality is limited.
Swaziland, a landlocked country, in the southern part of Africa, is amongst the countries without a pharmaceutical regulatory body [17]. Such a body is of paramount importance as it would help to ensure a regular supply of good quality medicines. Therefore, in the absence of such a body, the probability of any infiltration of sub-standard and counterfeit medicines into the distribution chain is high. Thus, the quality of medicines entering the country is not guaranteed. Therefore, this study aimed to assess the pharmaceutical equivalence and in-vitro bioequivalence of generic formulations of immediate release Nifedipine tablets available in Swaziland.
There are substantial evidences that the method of manufacture and the final formulation of the drug can markedly affect the bioavailability of the drug. In fact the World Health Organization (WHO) and all drug regulatory agencies support commercialization of generic medicines because they control costs and are irreplaceable therapeutic options in countries lacking the innovator product [18]. However, it is necessary to examine brand drug products in regard to in-vitro dissolution and in-vivo bioequivalence, and interchangeable uses with the innovator product. Therefore, in the present study in-vitro dissolution is used as a measure for bioequivalence of the four brands of Nifedipine tablets.
Materials and Methods
Nifedipine was obtained from Sigma Chemicals Co., USA. All other chemical reagents used were of pharmaceutical grade. All aqueous solutions were prepared exclusively in distilled water. Four different brands of Nifedipine 20mg immediate release tablets were obtain from retail pharmacy outlets and the Central Medical Store in Swaziland.
Assessment of parameters for pharmaceutical equivalence
Thin layer chromatographic identification: Thin layer chromatograms of pure Nifedipine (0.2 % w/v) in equal volumes of dichloromethane and methanol, and equivalent solutions of tablets obtained with ethylacetate: cyclohexane (2:3) as solvent system were compared using their Rf values and colour characteristics under ultraviolet (254nm).
Thickness and diameter: The thickness of the tablets was determined using vernier caliper and standard deviations were calculated. 10 tablets from each brand were used, and average values were calculated.
Uniformity of weight: Weight variation was determined by weighing 20 tablets from each brand individually, the average weight was calculated and the percentage variation of each tablet from the average weight of tablet was calculated [19].
Friability: The friability of the tablets was determined using 20 tablets from each brand, with a friability tester (Erweka TAR- 20) at a speed of 25rpm for 4min. The tablets were weighed before and after the friability test, and friability was determined as percent weight change [19].
Hardness: Hardness was determined by taking 10 tablets from each brand using a digital tablet hardness tester (TBH 210, Erweka) and the average of pressure (N) applied for crushing the tablet was determined [19].
Disintegration: Disintegration time was determined by taking 5 tablets from each of the brands and subjected into the disintegration tester (Manesty Tablet Disintegration Test Unit TD 75T176, England). The timer and the temperature (37 °C) were set, and Distilled water was used as the disintegrating medium. The time required to obtain complete disintegration of all the tablets was noted [19].
Drug content (Assay): 20 tablets were weighed from each of the brand, powdered and equivalent to 20 mg of Nifedipine were weighed and dissolved in sufficient quantity of methanol and make up to 100 ml with methanol. The solutions were suitably diluted with buffer solution pH 1.2 and the content of Nifedipine was estimated by measuring the absorbance in a spectrophotometer at 340nm using pH 1.2 as a blank [19].
Assessment of parameter for In-Vitro bioequivalence
Dissolution rate: In-vitro dissolution rate studies were carried out using dissolution apparatus type 2 [19], in 900 ml of 0.1 M Hydrochloric acid maintained at 37±0.5 °C. The stirring speed was set at 50rpm. At predetermined time intervals a 10- ml sample was withdrawn and replaced with fresh dissolution media up to 3hrs. After appropriate dilutions, the samples were analysed by the UV spectrophotometric method using a 2-cm layer cell at 340nm. Cumulative percent of drug released was calculated and the mean of six tablets each from the different brands was used in data analysis. The dissolution profiles for the different brands of Nifedipine tablets were generated after plotting the graph of amount of Nifedipine dissolved against dissolution time. The average T70 (time for 70% of the active drug to be dissolved) and the amount dissolved at 45 minutes were obtained for each brand.
Statistical analysis: All results obtained were subjected to statistical analysis of mean and standard deviation, using Microsoft Excel 2013 version.
Results
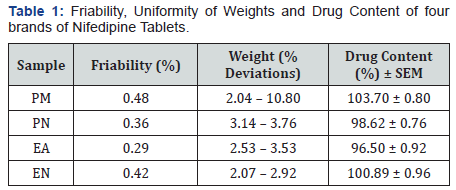
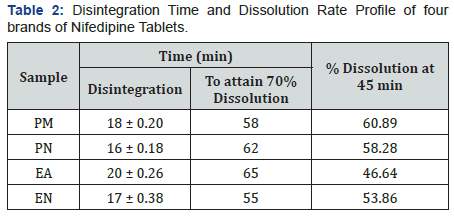
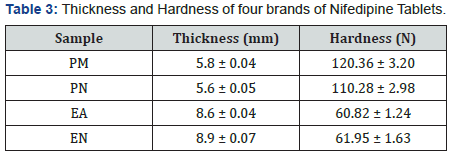
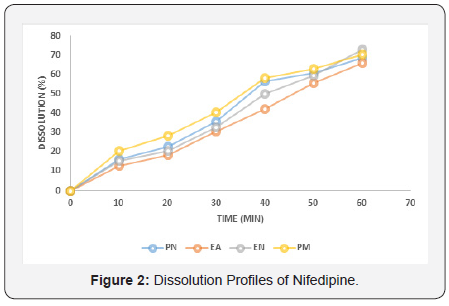
Immediate release Nifedipine 20mg tablets of different brands were subjected to various evaluation tests, such as thickness, uniformity of weight, disintegration time, drug content, hardness, friability and in-vitro dissolution. As summarized in Table 1, all the brands complied with the specifications for friability, as none as a mass loss greater than 1%, and for drug content, as the drug content of all brands ranged from 96.50 to 103.70 % [19]. Table 2 showed that none of the brands complied with the specifications for disintegration and in vitro dissolution. The dissolution profiles for the four brands is illustrated in Figure 2. Only two brands, PM and PN met the specification for diameter and thickness but failed the test for hardness (Table 3). Similarly, brands EA and EN met the specifications for hardness but failed the test for diameter and thickness (Table 3). The weight variations of the tablet (Figure 1), for three of the brands were between 2.07 and 3.76% which complied with pharmacopoeia specifications, indicating the presence of an acceptable amount of drug in these brands, however, brand PM failed the uniformity of weight test [19].
Discussion
Each of the immediate release Nifedipine brands had a shelflife of three years and the tablets were analysed at least a year before their expiry dates. Pure Nifedipine had Retardation factor (Rf) value of 0.72 and a similar spot was detected in all the tablet brands of Nifedipine. This preliminary test indicated that all the tablet brands contained the active ingredient, Nifedipine. One brand, PM did not comply with the specifications for weight uniformity [19], as the weights of more than two out of twenty tablets used for the test in these brands deviated from the mean value by an amount greater than 10.0 % (Table 1). This indicated that the tablets in the batch of this brand will vary in weights outside the official limits.
In the friability test as illustrated in Table 1, all the brands gave a weight loss of < 1% w/w, showing compliance with the official specification [19]. Hence, all the brands could withstand abrasion without loss of tablet integrity. Similarly, Table 1 showed that all the brands complied with the official specification for drug content of active drug content within the range 95-105 % [19]. This is an indication that all the brands when taken at the required doses are able to release the right amount of the active drug should elicit appropriate therapeutic response.
It is illustrated in Table 3, that only two out of the four brands met the specifications for hardness (Brands EA and EN), and the specification for thickness and diameter (Brands PM and PN). Both these parameters have an effect on the overall disintegration time and dissolution rate of the tablets. As indicated in Table 2, none of the brands met the specification of less than 15 minutes as disintegration time for uncoated tablets [19]. The inability of these brands to disintegrate within this time limit is an important indication that the tablets will not disintegrate in the gastrointestinal tract to release their contents into the system. Similarly, none of these brands (Table 2) met the specification for in-vitro dissolution test [19], which specifies that not less than 70% w/w labelled content should dissolve at 45 minutes. The dissolution rate profile showed that all the brands did not attain 70% dissolution in 45min period of determination and consequently had low (46.64 – 60.89%) percent dissolution at 45min. Consequently, these brands may exhibit poor bioavailability profiles in-vivo as well as poor clinical performances. The disintegration time and dissolution rates have direct bearing on the bioavailability profile of tablet dosage forms as it can be used to predict the drug release pattern in-vivo [11].
Bioequivalence of a generic product could be achieved either by in-vivo or in-vitro studies. In-vivo bioequivalence studies are frequently used to establish therapeutic equivalence but this approach is usually expensive and more rigorous and may require clinical trial or study expertise [20]. In-vitro dissolution profiles are proxies for establishing bioequivalence when the drug meets the criteria prescribed for a Biopharmaceutics Classification System (BCS) biowaiver [11,12]. The BCS considers three major factors- dissolution, solubility and intestinal permeability which influence the rate and extent of drug absorption from solid oral dosage forms [12]. The in-vitro and in-vivo bioequivalence correlation was launched in the ICH guidelines on Good Clinical Practice [21] and Guidance for Industry Bioavailability and Bioequivalence Studies [22], but this correlation is still debatable for some drugs, therefore, a complete and reliable bioequivalence study is the one that has data from both in-vitro and in-vivo studies.
While it is a known fact that generic drugs have the advantage of reducing medical expenses, a situation which is greatly desired in low-income countries like Swaziland, it is very essential to be doubly sure that these generic drugs are of good quality, especially in terms of their safety and bioavailability. This will ensure that patients with life-threatening diseases like hypertension, are adequately treated to improve their quality of lives. This study has demonstrated that while the four different brands of immediate release Nifedipine 20mg tablets that are commercially available in Swaziland contained appropriate Nifedipine drug content, they cannot be said to be pharmaceutically equivalent, and they may demonstrate poor bioavailability, and hence inability to elicit the desired therapeutic responses in patients receiving them for the management of hypertension or angina pectoris.
Conclusion
Results from this study have shown that none of the brands is of acceptable quality, and the brands are not pharmaceutically equivalent. Moreover, none of the brand is likely to produce acceptable oral bioavailability, which is deduced from in-vitro bioequivalence. Thus, switching or substituting brands of immediate release Nifedipine for patients should be guided by a critical assessment of the dissolution rate and other quality parameters.
Acknowledgement
The authors are grateful to the management and staff of Swaziland Christian University for providing the analytical reagents for the study and for allowing the use of the laboratory in the Department of Pharmacy for the analytical work.
References
- Cooper RS, Kaufman JS, Bovet P (2017) Global Burden of Disease Attributable to Hypertension. JAMA 317(19): 2017-2018.
- Furberg CD, Psaty BM, Meyer JV (1995) Nifedipine. Dose-related increase in Mortality in patient with coronary heart disease. Circulation 92(5): 1326-1331.
- Poole-Wilson PA, Kirwan BA, Vokó Z, de Brouwer S, van Dalen FJ, et al. (2006) The Safety of Nifedipine GITS in stable Angina: The Action trial. Cardiovasc Drugs. 20(1): 45-54.
- (2005) Multisource (generic) products: Guidelines on registration requirements to establish interchangeability. World health organization (WHO), Geneva, Switzerland. p: 1-39.
- Richard Finkel, Michelle A Clark, Luigi X Cubeddu (2009) Lippincott’s Illustrated Reviews. Pharmacology, (4th Edn). pp: 878.
- Newton PN, Green MD, Fernández FM (2010) Impact of poor quality medicines in the developing world. Trends Pharmacol Sci 31(3): 99- 101.
- Woodcock J (2004) The concept of Pharmaceutical quality. America Pharmaceutical Review 47(6): 1-3.
- (2017) WHO Global Surveillance and Monitoring System for substandard and falsified medical products. World Health Organization, Geneva, Switzerland. p: 73.
- (2007) Quality Assurance of Pharmaceuticals volume.2. World Health Organization, Geneva, Switzerland. pp: 418.
- Nguyen TA, Knight R, Roughead EE, Brooks G, Mant A (2015) Policy options for pharmaceutical pricing and purchasing: issues for low- and middle-income countries. Health Policy Plan 30(2): 267–280.
- Amidon GL, Lennernäs H, Shah VP, Crison JR (1995) A theoretical basis for a biopharmaceutics drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 12(3): 413-420.
- Cook JA, Bockbrader HN (2002) An Industrial Implementation of the Biopharmaceutics Classification System. Dissolution Technol 9(2): 6–8.
- (2015) Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research FDA/CDER. p: 1-17.
- Oyeniyi YJ, Ayorinde JO (2012) Pharmaceutical Evaluation of some commercial brands of Nifedipine (20mg) sustained release tablets, Marketed in commercial city of Kano. IJBPAS 1(4): 585-593.
- Sheeba FR (2009) Formulation & Evaluation of Nifedipine sublingual tablets. Asian Journal of Pharmaceutical &clinical research 2(3): 1-5.
- Mehta KA, Kislalioglu MS, Phuapradit W, Malick AW, Shah NH (2002) Multi-unit controlled release system of Nifedipine & Nifedipine: pluronic F-68 solid dispersion: Characterization of release mechanisms. Drug Dev Ind Pharm 28(3): 275-285.
- (2004) Report on Technical Support on Drug Regulatory Mechanisms and ART Expansion, BK Botwe, Ministry of Health, WHO Consultant.
- (2017) A study on the public health and socioeconomic impact of substandard and falsified medical products. World Health Organization, Geneva, Switzerland. p: 77.
- (2007) Metformin monograph, USP 29-NF 24 Pharmacopeia Forum. United States Pharmacopoeia 31(4): 1365.
- Peters JR, Hixon DR, Conner DP, Davit BM, Catterson DM, et al. (2009) Generic drugs-safe, effective and affordable. Dermatol Ther 22(3): 229- 240.
- (2015) ICH: Harmonized Guideline for Good Clinical Practice. E6 (R2).
- (2014) Guidance for Industry Bioavailability and Bioequivalence Studies submitted in NDAs or INDs — General Considerations U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research FDA/CDER. p: 1-29.






























