Bioequivalence Study of Morphine Sulfate CR Tablets
Aslihan Arslan, Burcu Bulut, Onur Pinarbaşli, Yilmaz Çapan* and Hatice Öncel
Ilko Ilaç San ve Tic AŞ, Üniversiteler Mah, Turkey
Submission: April 04, 2018; Published: April 20, 20188
*Corresponding author: Yýlmaz Çapan, Üniversiteler Mah 1596 Cadde, Hacettepe Üniversitesi Beytepe Kampüsü Teknokent ülko Argem Binasý, Beytepe Çankaya, Ankara/Türkiye, Tel: +903122486800; Email: ycapan@ilko.comtr
How to cite this article: Aslýhan A, Burcu B, Onur P, Yýlmaz Ç, Hatice Ö. Bioequivalence Study of Morphine Sulfate CR Tablets. Mod Appl Bioequiv Availab. 2018; 3(5): 555623. DOI: 10.19080/MABB.2018.03.555623
Abstract
Purpose: To investigate the bioequivalence of Morphine Sulfate 100mg Controlled Release (CR) Tablets.
Method: Open-label, single dose, 4-period crossover, randomized design in 48 healthy subjects under fasting and fed conditions. Test product; Morphine Sulfate 100mg Controlled Release Tablets (Ilko tlaf San ve Tic As Turkey, batch no: 1406229001) was compared with the corresponding tablet strength of the reference product MS Contin® 100mg Controlled Release Tablets (Purdue Pharma LP, USA, batch no: WJM31) under fasting and fed conditions. The statistical method is testing for Cmax, AUC0-t and AUC0-∞ . Bioequivalence study was based upon the 90% confidence interval (90%CI) for the test and reference geometric mean ratio. Bioequivalence study was to be assumed when the 90%CI fell within the recommended acceptance interval 80%; 125%.
Results: The test and reference geometric mean ratio and 90%CI for morphine sulfate were as follows: Fasting study- 99.08% (92.85%- 105.74%), 101.93% (97.73%-106.32%) and 103.60% (99.27%-108.12%) for Cmax, AUC0-t and AUC0-∞, respectively. Fed study-88.93% (82.96%- 95.34%), 95.30% (88.90%-102.16%) and 96.55% (90.41% - 103.11%) for Cm”AUC0-t and AUC0-∞, respectively. For both test and reference product a food interaction is observed. The food effect is more pronounced for the reference product, compared with the test product.
Conclusion: Pharmacokinetic parameters are in the acceptance range of 80%; 125% for both fed and fasting bioequivalence studies.
Keywords: Morphine sulfate; Bioequivalence; Controlled release, Test product; Reference product; Food effect
Abbreviations: AUC0-t =AUC0-tlast : Area Under The Plasma Concentration Curve from Administration to Last Observed Concentration At Time t; AUC0-∞ : Area Under the Plasma Concentration Curve Extrapolated to infinite Time; Cmax: Maximum Plasma Concentration; tmax: Time Until Cmax is Reached; t1/2(λ) [h]: = Terminal Half Life Calculated From λ according to t1/2 = ln(2)/ λ; λ [h-1]: = Terminal Rate Constant in h-1 Calculated by Log- Linear Regression of the Concentrations Observed During the Terminal Phase of Elimination; CR: Controlled Release; 90% CI: 90% Confidence Interval; Clast: Concentration at the Quantifiable Point; tlast: Last Time Point with Quantifiable Concentration; OTC: Over The Counter; CRF: Case Report Form; LLOQ: Lover Limit of Quantification; LC-MS/MS: Liquid Cromatography-Mass Spectrometry; WHO: World Health Organisation; TÜBlTAK: The Scientific and Technological Research Council of Turkey; TMO: Turkish Grain Board
Introduction
Cancer is the second leading cause of death worldwide. In 2015, cancer caused more than 8.7 million deaths worldwide and is the second leading cause of death after cardiovascular disease [1,2]. According to the World Health Organization (WHO) reports that it will be a substantive increase to 19.3 million new cancer cases per year by 2025, due to growth and ageing of the global population. More than half of all cancers (56.8%) and cancer deaths (64.9%) in 2012 occurred in less developed regions of the world, and these proportions will increase further by 2025 [3].
Pain from cancer is a major health care problem. Every three cancer patients suffer from moderate/severe cancer pain. 30 percent of cancer patients have pain during the diagnosis and they have 65 to 85 percent pain when the disease is advanced [4]. The World Health Organization (WHO) pain relief ladder is long- used guideline for the treatment of cancer-related pain. Cancer pain be managed in a stepwise progression recommended by the ladder, moving from nonopioids and NSAIDs (Step 1) to “weak” opioids (Step 2) and then to “strong” opioids (Step 3). A number of studies have addressed the effectiveness of this stepwise approach to pain treatment [5].
Morphine is a well-known opioid derivative of pharmacokinetic and pharmacodynamic properties that is used orally, intravenously, intramuscularly, and subcutaneously in the palliative treatment of moderate to severe cancer pain [4,5]. Morphine is found in the 19th WHO Model List of Essential Medicines as different dosage forms like that tablet, extended release tablet, injection, liquids, granules [6]. Despite this situation, an estimated 5.6 billion people in the world live in countries with low levels of, or non-existent access to opioid analgesics for treatment of moderate to severe pain (80% of the world population) [7].
Cancer is a growing public health problem in Turkey, as well as worldwide. However, there is no reference and generic products of morphine in Turkey markets. İlko llaí San ve Tic. AŞ developed Morphine Sulfate Immediate and Controlled Release Tablet formulations. Morphine sulfate was provided from Turkish Grain Board (TMO) which produce morphine sulfate according to the American and European Pharmacopoeia standards. New generic formulations of Morphine sulfate controlled release 100mg, 60mg, 30mg and 15mg tablets were developed having the same composition original product, MS Contin® Controlled Release 100mg, 60mg, 30mg and 15mg Tablets (Purdue Pharma L.P., USA).
MS Contin® Controlled Release Tablets and our jeneric product contain morphine sulfate in a dual-control polymer matrix that consists of hydroxyethyl cellulose and a higher aliphatic alcohol. These two hydrophobic matrix were provide to slow the diffusion of drug into the aqueous phase, which limits diffusion into the gastrointestinal tract and absorption into the body [8].
After the formulation studies; in vitro tests, stability tests and pilot production studies were carried out. The bioequivalence studies were carried out after the in vitro tests were successful. A single dose of Morphine Sulfate 100mg Controlled Release Tablets (Ilko llaf San ve Tic A£ Turkey, batch no.: 1406229001) and MS Contin® 100mg Controlled Release Tablets (Purdue Pharma L.P., USA, batch no.: WJM31) were evaluated in bioequivalence studies. The test product and the reference product were administered under fed and fasting conditions. The pharmacokinetics of Morphine sulfate was evaluated in 48 healthy male volunteers. The purpose of these studies was to demonstrate the bioequivalence of test and reference products of Morphine Sulfate 100mg Controlled Release Tablets. Also to compare the pharmacokinetic results in fed and fasting conditions and to research food effect on morphine bioavailability.
Materials and Methods
Study design
The present study consisted of open, randomized, four- period cross-over, single dose pharmacokinetic trial in 48 healthy male volunteers. In study, the bioavailability of morphine following a given Morphine Sulfate 100mg Controlled Release Tablet, each controlled release tablet contains 100mg morphine sulfate (Test) was compared with the corresponding tablet strength of the original product (Reference), under fasting and fed conditions. The test product and reference product were administered to 48 healthy male volunteers. Subjects received each of the treatments either in period 1 and period 2 (fasting condition) or in period 3 and period 4 (fed condition) according to a predetermined randomization scheme, treatments consisting of either Morphine Sulfate 100mg Controlled Release Tablets (test product) or MS Contin® 100mg Controlled Release Tablets (reference product), both containing 100mg morphine (as morphine sulfate). Treatments were separated by a washout phase of seven days between the periods, according to the (at least) one-week period required in the Study Protocol. A naltrexone blockade should be used to remove the risk of any opioid-related adverse events. Naltrexone should be administered well in advance of dosing to achieve adequate blockade of opioid receptors. So all enrolled subjects were administered single oral doses of 50mg naltrexone (as naltrexone hydrochloride) together with 240ml tap water 10 hours pre-morphine dose, 1 hour premorphine dose and 12 hours post-morphine dose on Day 0 and Day 1 in four consecutive periods. In case of nausea despite naltrexone administration, ondansetron was given by investigator's judgement and according to the Study Protocol to prevent vomiting. The given drug and time were recorded in the CRF. For single dose pharmacokinetics, blood samples were collected up to 48 hours after drug administration. Safety evaluations were done by adverse event assessments, pulse rate/ blood pressure measurements and ECG readings. Subjects were hospitalized for about 27 hours during each period (discharge from the clinical unit on Day 1, approx. 13 hours after drug administration. Alcohol consumption was not allowed from two days prior to drug administration at Erciyes University-Hakan Çetinsaya İKU ve Araştirma Merkezi (Center for GCP) until the last blood sampling of each period. Smoking was not allowed for the time periods of blood sampling.
Fasting periods
After intake of an evening snack (total caloric value of approx. 600kcal) at approx. 07:30pm. On the day of admission, the subjects fasted overnight for a minimum of 10 hours. During this fasting time, liquid intake was limited to tap water which was allowed to be consumed up to approx. 1.5l for the days of dosing, apart from one hour before until one hour after drug administration, when only the water supplied with the drug and that kind of liquid consumed during the high-fat breakfast was permitted. A high-fat breakfast (total caloric value of approx. 967.28kcal) was provided 30min before dosing in period 3 and period 4 and had to be finished 5 minutes before dosing. After dosing, fasting continued for 4 hours after drug administration. At the end of this fasting period a standard lunch (total caloric value of approx. 1200kcal) was supplied, followed by a standard dinner (total caloric value of approx. 1200kcal) served 10 hours after drug administration.
Diet and Dietary Restrictions
In addition to the high-fat breakfast consumed during period 3 and period 4, all subjects were provided with standardised meals throughout the hospitalization period. Except for xanthine-and alcohol-containing foods or beverages (including orange and grapefruit products) which were not allowed during confinement, the diet consisted of a normal dietary plan. No foods and beverages containing caffeine or other methylxanthines (coffee, tea, coke, chocolate) and fruit juice were allowed from 2 days prior to each dosing until the last blood sampling of either period. No grapefruit and orange products were allowed from 7 days prior to the first dosing until the last blood sampling. The chewing of chewing gum was not allowed on the days of dosing. Subjects were not allowed to take prescribed systemic or topical medications beginning 2 weeks and no OTC medications (including herbal remedies) beginning 1 week prior to the start of the study. Subjects were furthermore required not to use either systemic or topical drugs (including herbal remedies) until after completion of the study. Treatment with any investigational drug (i.e. drug not yet approved) in the last two months before beginning of the trial was not allowed. In the case of intake or administration of any prescribed systemic or topical medication within 4 weeks before the start of the study because of an insignificant illness, this should have been stated in the CRF.
Blood samples
Pharmacokinetic blood withdrawals were performed at the following relative study times of Day 1-Day 3 in each period: 0h (pre-dose), 30min (0.50h), 1h, 1h 30min (1.50h), 2h, 2h 30min (2.50h), 3h, 3h 30min (3.50h), 4h, 5h, 6h, 8h, 10h, 12h, 24h, 36h and 48 hours post-dose.
Study population
Participants were recruited from the subject pool of Erciyes University Hakan Çetinsaya lKU ve Arajtirma Merkezi (Center for GCP). A total number of 72 male Caucasian subjects were screened by physical examination and clinical laboratory tests. 48 subjects were included in the study. Altogether 44 subjects (per protocol population), at an age of 21-45 years and with a BMI range of 19.6-29.6kg/m2, terminated the study regularly Plasma samples of 44 completed cases for each treatment were available for analysis of morphine concentrations. Mean (±S.D.) demographic data for 44 completed cases were 32.2 (±7.4) years, 76.4(±10.1)kg weight, 176.0 (±8.0)cm height and 24.6 (±2.4)kg/ m2 for BMI. All individual demographic data of subjects including calculation of descriptive statistics are tabulated in the Clinical Performance Report.
Statistical analysis
Determination of 90% confidence intervals for the difference in the mean values of the test and the reference product for the primary target parameters in the logarithmic domain.
Calculation of Sample Size
According to the literature; the intrasubject variability of AUC and Cmax is estimated to be about in the range of 20%- 25% or even higher. In order to demonstrate bioequivalence with a power of 80% and a test/reference parameter ratio of geometric means between 0.95 and 1.05, a total number of at least 44 completed cases (per protocol population for pharmacokinetic evaluation) has been calculated to be necessary for a bioequivalence conclusion.
In-Vitro testing
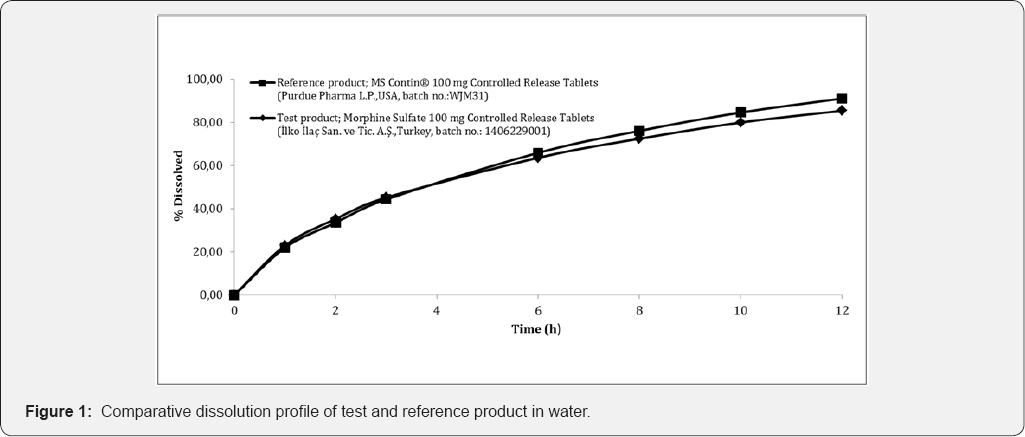
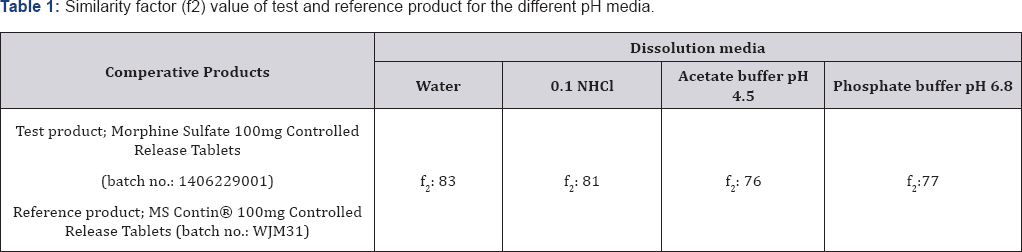
Comparative dissolution studies at different dissolution media were performed to demonstrate in vitro equivalence between for the reference products and test product. The dissolution test were carried out with a Distek Evolution 6300 dissolution tester. It used dissolution method recommended by the FDA which is Apparatus 1 (Basket), a volume of 900mL water (deaerated) at 37.0±0.5 °C. The baskets worked for 12 hours at 50rpm. Each sample was collected at 1, 2, 3, 6, 9 and 12 hour time points. In addition to water media, dissolution sudies were carried out at three buffer solutions (pH 1.2; pH 4.5; pH 6.8). The dissolution profiles obtained were evaluated by similarity factor (f2) [9].
In-Vivo testing
Pharmacokinetic analysis: For each treatment (test product: Morphine Sulfate 100mg Controlled Release Tablet, batch no.: 1406229001; reference product: MS Contin® 100mg Controlled Release Tablets, batch no.: WJM31) and each volunteer participating in this open, randomized, single dose, 4-period crossover bioequivalence study under fasting and fed conditions, the following pharmacokinetic parameters will be calculated for morphine, using period-related product assignments as follows: T1, test product, fasting; T2, test product, fed; R1, reference product, fasting; R2, reference product, fed.
Primary pharmacokinetic parameters for morphine: AUC0-tlast [ng*h/mL]: = area under the plasma concentration vs time curve in ng*h/ml, calculated by the linear trapezoidal rule based on plasma concentrations following drug administration (time 0h) up to the time (tlast) of the last quantifiable concentration. Initial values below LLOQ will be set to zero for AUC0-tlast computation Cmax [ng/mL]: = observed maximum concentration in ng/ml.
Secondary pharmacokinetic parameters for morphine: AUC0-∞ [ng*h/mL]: = area under the plasma concentration vs time curve in ng*h/ml, calculated by extrapolation from time 0h to infinity using the following equation:
AUC0-∞= AUC0 -tlast + AUCtlast-∞
tmax [h]: = observed time in h after dosage to reach Cmax
t1/2(λ) [h]: = terminal half life calculated from λ according to t1/2=ln(2)/ λ
λ [h-1]: = terminal rate constant in h-1 calculated by log-linear regression of the concentrations observed during the terminal phase of elimination
Auxiliary pharmacokinetic parameters for morphine: AUC%-extrapol [%]: = percentage of the residual area ( AUC0-tlast ) extrapolated to infinity in relation to AUC0_∞ to be calculated as 100*( AUC0-tlast/AUC0_∞ AUC0-tlast [ng*h/ml]: = residual area under the plasma concentration vs time curve in ng*h/ml, calculated according to AUC0-tlast = Clast / λ where Clast is the last measured value above LLOQ of the terminal slope at tlast]
Clast [ng/ml]: = concentration at the last quantifiable point (c≥LLOQ)
tlast [h]: = last time point with quantifiable concentration (c≥LLOQ)
For pharmacokinetic calculations, the program package Phoenix WinNonlin, version 6.3 as well as Excel version 2003 (or upgraded version) will be employed. Individual values of pharmacokinetic parameters and their descriptive statistics will be tabulated, except for Clast , tlast and the residual area AUCtlast -∞ , which will be used for calculation of the extrapolated area AUC0_∞ . All statistical evaluations of this 4-period crossover study will be performed by two-way crossover comparisons T1 vs R1 (fasting condition), T2 vs R2 (fed condition), T1 vs T2 (food effect for test product) and R1 vs R2 (food effect for reference product).
Results and Discussion
In Vitro result
The dissolution profiles were compared; the dissolution profiles obtained were evaluated by similarity factor (f2) [9]. According to the EMEA and FDA Guidelines; Dissolution similarity may be determined using the J2 statistic as follows:
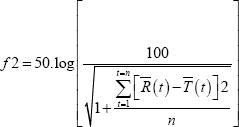
In this equation J2 is the similarity factor, n is the number of time points, R(t) is the mean percent reference drug dissolved at time t after initiation of the study; T(t) is the mean percent test drug dissolved at time t after initiation of the study. For both the reference and test formulations, percent dissolution should be determined [9].
An f2 value between 50 and 100 suggests that the two dissolution profiles are similar. Comparative dissolution profile of test and reference product in water is shown in Figure 1. The results obtained confirmed that, for different dissolution media under comparison, there was a similar in vitro dissolution profile because of that f2 value of all dissolution media is higher than 50 ( Table 1).
In Vivo result
In these studies, we investigated the bioequivalence of test and reference products of Morphine Sulfate 100mg Controlled Release Tablets. According to the EMEA and FDA Bioequivalence Guideline; two medicinal products containing the same active substance are considered bioequivalent if they are pharmaceutically equivalent or pharmaceutical alternatives and their bioavailabilities (rate and extent) after administration in the same molar dose lie within acceptable predefined limits. These limits are set to ensure comparable in vivo performance, i.e. similarity in terms of safety and efficacy [9].
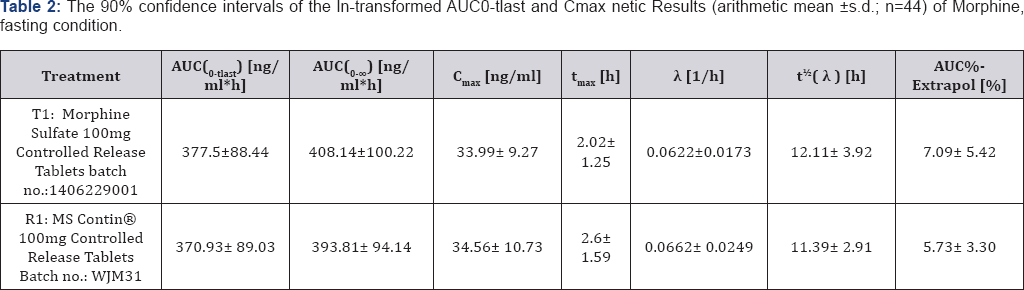
Based on the results of the present study, both the extent (AUC0-tlast) and rate (Cmax) of bioavailability of the test product (Morphine Sulfate 100mg Controlled Release Tablet, batch no.: 1406229001) and the reference product (MS Contin® 100mg Controlled Release Tablets, batch no.: WJM31) are comparable under fasting and fed conditions. The 90% confidence intervals of the ln-transformed AUC0-tlast and Cmax meet the bioequivalence criteria of 80%-125% as laid down in the Study Protocol. Pharmacokinetic Results (arithmetic mean±s.d.; n=44) of Morphine, fasting condition is shown in Table 2 and Pharmacokinetic Results (arithmetic mean±s.d.; n=44) of Morphine, fed condition is shown in Table 3.
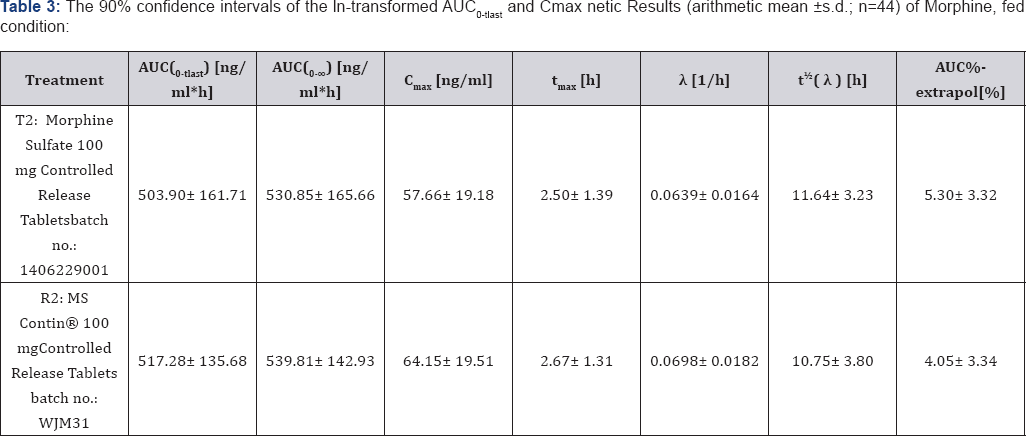
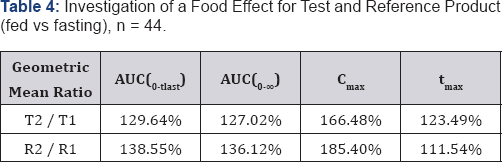
For both test and reference product a food interaction is observed according to provisions given in the Study Protocol, because 90% confidence intervals of primary target parameters AUC0-tlast and Cmax fail to meet the 80%-125% acceptance range for the fed vs fasting comparison. Rate and extent of drug absorption are higher under fed condition, irrespective of the study preparation concerned. The food effect is more pronounced for the reference product, compared with the test product. A clinically relevant difference in the tolerability and safety of the treatments was not detected. Result of a Food Effect for Test and Reference Product (fed vs fasting), n = 44 is shown in Table 4.
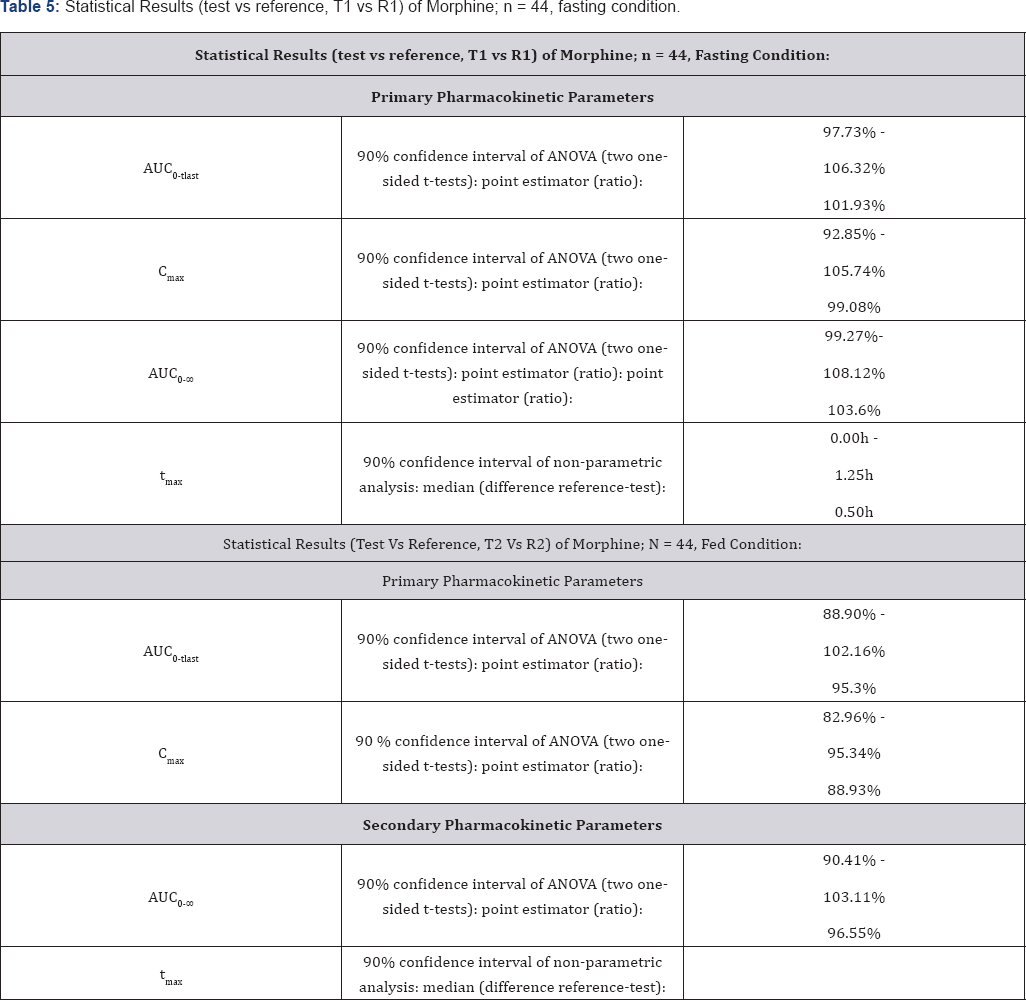
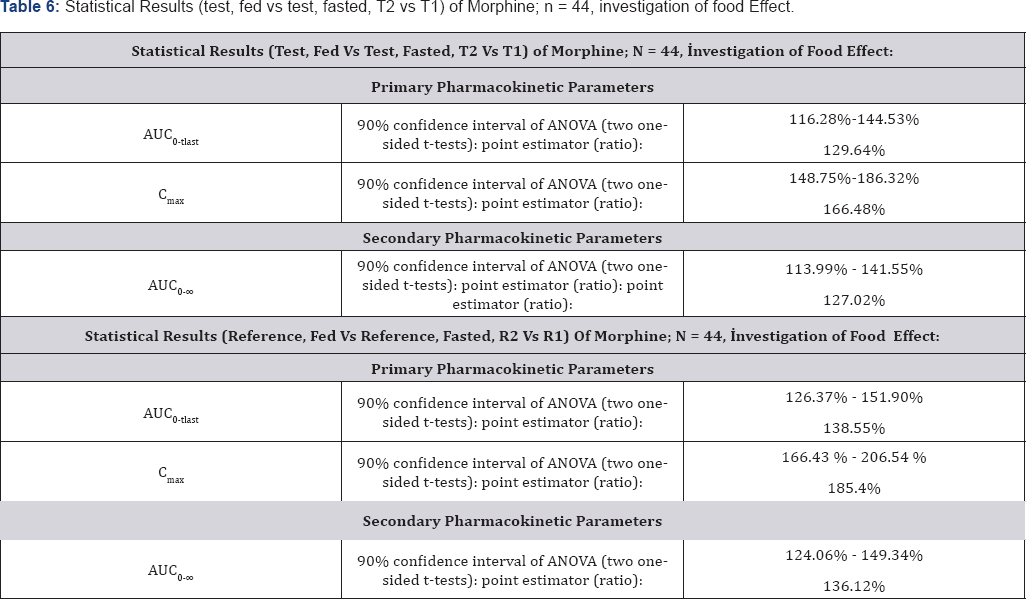
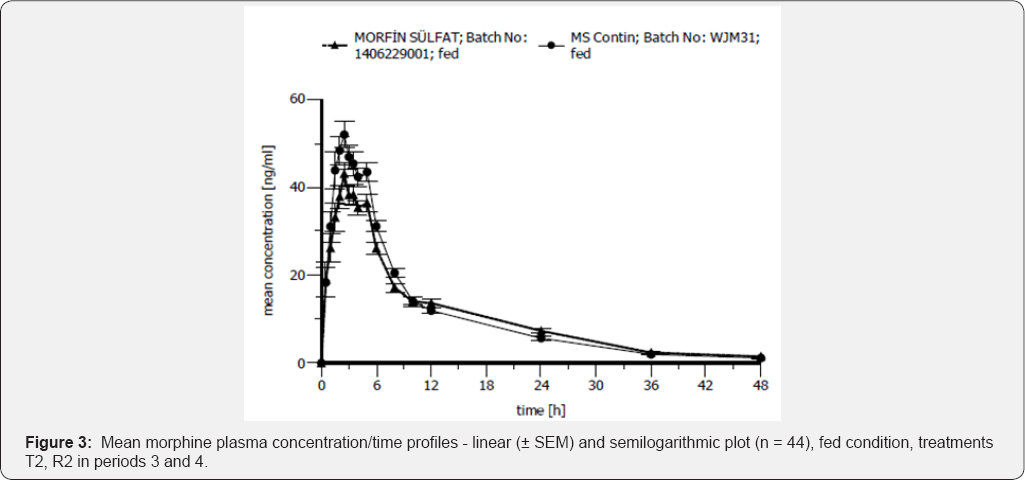
Bioequivalence between Morphine Sulfate 100mg Controlled Release Tablets and MS Contin® 100mg Controlled Release Tablets is demonstrated for primary target parameters (AUC0- tlast, Cmax) in 44 healthy male Caucasian subjects after single dose administration under fasting and fed conditions. Results are shown in Table 5 & 6. Mean morphine plasma concentration/ morphine plasma concentration/time profiles-linear (±SEM) time profiles-linear (±SEM) plot (n=44), fasting condition, plot (n=44), fed condition, treatments T2, R2 in periods 3 and 4 treatments T1, R1 in periods 1 and 2 is shown in Figure 2. Mean is shown in Figure 3.

Conclusion
The 90% CI of Morphine sulfate for Cmax , AUC0-t and AUC0-∞ were within 80.00-125.00% for both fed and fasted bioequivalence studies. Therefore
a) The generic formulation of Morphine sulfate Controlled Release 100mg Tablets was bioequivalent with the innovator formulation of MS Contin® Controlled Release 100mg Tablets (Purdue Pharma L.P., USA) under fasted conditions.
b) The generic formulation of Morphine sulfate Controlled Release 100mg Tablets was bioequivalent with the innovator formulation of MS Contin® Controlled Release 100mg Tablets (Purdue Pharma L.P., USA) under fed conditions.
Acknowledgement
We would like to acknowledge İDE llaí Ruhsatlandirma, Biyofarmasötik, Danişmanlik, Eğitim ve Mým. Hiz Diş Tic İth İhr Ltd Şti for CRO; Erciyes University Hakan Çetinsaya İKU ve Araştirma Merkezi (Center for GCP) for clinical study; Analytisches Zentrum Biopharm GmbH for Analytical study, Pharmakin Consulting Services UG for Biometrics/Reporting. This research was supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK), Hacettepe University and Hacettepe University Faculty of Pharmacy.
References
- GBD 2015 Mortality and Causes of Death Collaborators (2016) Global, regional, and national life expectancy, all-cause mortality, and cause- specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053): 1459-1544.
- Global Burden of Disease Cancer Collaboration (2017) Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability- Adjusted Life-years for 32 Cancer Groups, 1990 to 2015 A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 3(4): 524-548.
- WHO Press Release N 223 (2013) Latest world cancer statistics Global cancer burden rises to 14.1 million new cases in 2012: Marked increase in breast cancers must be addressed. World Health Organization, France.
- Levy MH (1996) Pharmacologic Treatment Of Cancer Pain. N Engl J Med 335(15): 1124-1132.
- Cleary JF (2007) The Pharmacologic Management Of Cancer Pain. J Palliat Med 10(6): 1369-1394.
- Annex 1 (2015) 19th WHO Model List of Essential Medicines.
- Forte G (2015) WHO, Coordinator Policy, Acces and Use Essential Medicines and Health Products (2015) Access to controlled medicines. World health organization,Switzerland.
- Amabile CM, Bowman BJ (2006) Overview of Oral Modified- Release Opioid Products for the Management of Chronic Pain. Ann Pharmacother 40(7-8): 1327-1335.
- (2010) Guideline On The Investigation Of Bioequivalence. EMEA CPMP/EWP/QWP/1401/98 Rev, European Medicines Agency, London, USA.






























