Pelvic External Myofascial Mobilization Improves Both the Short- and Long-Term Outcomes of Chronic Pelvic Pain
Ajimsha MS1*, Ahmad Majzoub2,3, Pramod D Shenoy4, Laith Ahmad Ismail4 and Smithesh Kooven4
Submission: February 2, 2024; Published: May 10, 2024
*Corresponding author: Ajimsha MS, Qatar Rehabilitation Institute, Hamad Medical Corporation, Qatar
How to cite this article: Ajimsha MS*, Ahmad Majzoub, Pramod D Shenoy, Laith Ahmad Ismail and Smithesh Kooven. Pelvic External Myofascial Mobilization Improves Both the Short- and Long-Term Outcomes of Chronic Pelvic Pain. J Yoga & Physio. 2024; 11(2): 555811 DOI:10.19080/JYP.2024.11.555811
Abstract
Objective: To evaluate the short- and long-term effects of a four-session pelvic external myofascial mobilization (PEMM) for managing chronic pelvic pain syndrome (CPPS) in males.
Methods: A retrospective chart review was conducted for male CPPS patients who underwent PEMM therapy at Qatar’s Hamad Medical Corporation from January 1st, 2019, to January 3rd, 2021. Follow-up (FU) evaluations were conducted after 9 months. The National Institutes of Health-Chronic Prostatitis Symptom Index (NIH-CPSI) and numerical rating scale (NRS) were utilized to measure patient-rated symptoms and pain at defined intervals.
Results: A total of 29 patients who completed the PEMM therapy and FU were included for analysis. The baseline NIH-CPSI score (30.5 ± 6.1) decreased by 74.3% immediately post-treatment and remained mostly unchanged (69.8%) at the FU. The NRS score also showed a significant reduction in pain (P < 0.001). Although the NIH CPSI sub-scores for pain and quality of life were unchanged at FU, a negative change was found in the post-hoc pairwise analysis of the CPSI total and urinary symptom sub-scores when compared to the post-test.
Conclusions: Closed protocol PEMM therapy led to significant symptom improvement with effects comparable to other soft tissue-biofeedback-cognitive therapy-based studies but with superior results. The relative retention of effects in the pain and quality-of-life domains at FU is promising. PEMM therapy is an effective non-invasive treatment for CPPS, with short- and long-term improvements in symptoms and pain.
Impact: This study has important implications for physical therapy, urology, and CPPS communities. Brief four-session PEMM therapy is an effective, external and non-invasive option for CPPS, with short- and long-term improvements in symptoms and pain. It has the potential to improve quality of life and reduce the cost and burden of care for CPPS. Further studies with control groups are needed to confirm these findings and identify better responding CPPS phenotypes.
Keywords: Chronic Prostatitis (CP); Chronic Pelvic Pain Syndrome (CPPS); Fascial Connectivity; Myofascial Mobilization; Pelvic Floor Physical Therapy (PFPT)
Abbreviations: CP: Chronic prostatitis; CPPS: Chronic pelvic pain syndrome; PEMM: Pelvic External Myofascial Mobilization; BPH: Benign Prostatic Hypertrophy; LUTS: Lower Urinary Tract Symptoms; UTI: Urinary Tract Infection; PFM: Pelvic Floor Muscle; PFPT: Pelvic Floor Physical Therapy; EMM: External Myofascial Mobilization; PMM: Pelvic Myofascial Mobilization; AOS: Anterior Oblique Sling; EO: External Oblique; IO: Internal Oblique; HA: Hip Adductor; AAF: Adductor-Abdominal Fascia; POS: Posterior Oblique Sling; LD: Latissimus Dorsi; GMx: Gluteus Maximus; TLF: Thoracolumbar Fascia; PSIS: Posterior Superior Iliac Spine; NIH-CPSI: National Institute of Health - Chronic Prostatitis Symptom Index; QoL: Quality of Life; NRS: Numeric Rating Scale; PRE: Pre-test; POST: Post-test; FU: Follow Up
Introduction
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a debilitating medical condition that affects 2%-16% of men globally and accounts for 90% of prostatitis-related outpatient visits [1,2]. While it is the third most prevalent genitourinary diagnosis in males overall, trailing only benign prostatic hypertrophy (BPH) and prostate cancer [3], and is the most encountered condition in men under the age of 50 [4]. Although once thought to be a condition of prostate infection and inflammation, CP/CPPS in men is now described as persistent idiopathic pelvic pain or discomfort that is often associated with lower urinary tract symptoms (LUTS), sexual dysfunction, and psychosocial challenges [5]. CPPS has a grave repercussion on quality of life and can cause economic hardships [2], with loss of employment reported in 25% of males and a decrease in leisure activities in 50% [6]. CPPS is distinguished by symptoms lasting ≥3 months during the preceding 6 months in the absence of bacterial urinary tract infection (UTI) [7].
CP/CPPS is currently believed to be a more complex disorder having negative influences on the neuromuscular, autonomic, and brain-level systems [5]. Because of that, emphasis on adequate evaluation of CP/CPPS patients using the “UPOINT” phenotype classification system has been proposed in attempt to offer a multidisciplinary symptom-based therapeutic strategy [7]. Furthermore, this multimodal strategy of treatment has been endorsed by international societies and is believed to be associated with the best clinical outcomes [8].
Pelvic floor muscle (PFMs) tenderness or the myofascial phenotype is highly prevalent in men with CP/CPPS and is currently believed to be an important cause of chronic pelvic pain as the associated increase in intrapelvic pressure may exacerbate prostate or bladder symptoms [9]. In the majority of cases, palpating tender muscular areas will replicate the patient’s discomfort in a discernible pattern [10]. As such, pelvic floor physical therapy (PFPT) is one of the non-pharmacologic treatment methods that can be used in this patient population. Several PFPTs are available including soft tissue releases [11], therapeutic exercises [12], biofeedback [13], electricalthermal modalities [14], extracorporeal shock wave therapy [15], and neuromodulation [16]. These treatments have found to be beneficial in more than 70% of men failing conventional pharmacologic therapy [17].
“Pelvic myofascial mobilization (PMM) entails internal or external mobilization of soft tissues in and around the pelvis. External Myofascial Mobilization (EMM) focuses on effectively managing fascial dysfunction by utilizing the latest knowledge of fascial architecture, myofascial connectivity [18], and force transmission [19]. Through individualized care, EMM aims to optimize therapeutic outcomes and improve overall well-being by addressing the specific needs of each person [20]. Internal PMM, on the other hand, mobilizes pelvic floor muscles through the rectum [21]. An application that is supported by research [17,21], however, is not commonly practiced due to patients’ discomfort or in some circumstances, cultural unacceptance [20]. Despite its prevalence, the CPPS myofascial pattern is still underappreciated and underrated both in clinical practice and in medical literature [22].
Furthermore, many urologists are unaware of the importance of evaluating pelvic floor myofascial dysfunction in men with complex CP/CPPS. While a few trials have shown that the PMM is effective in treating the muscle tenderness phenotype of CP/CPPS, reports of long-term effects are scarce [22-25]. Therefore, the aim of this work is to determine both short- and long-term effects of a closed protocol four session PEMM program in the management of CPPS in males.
Material and Methods
This was a retrospective chart review conducted at the multidisciplinary pelvic pain unit of the department of Urology at Hamad Medical Corporation in Qatar. With a waiver of written informed consent, the HMC Research Ethics Board approved the study without any ethical concerns. Data of male patients older than 20 years, referred to PFPT between Jan 2020 and July 2021, with a diagnosis of myofascial CPPS were short-listed for inclusion in the study. Patients with a history of CPPS for more than six months who completed a four-session PEMM program and in whom follow-up evaluation data was available were included in this study. Men with definite/organic causes for pelvic pain, such as previous surgeries, ongoing genitourinary infections, trauma or malignancy were excluded. Furthermore, patients with pathological causes of voiding dysfunction including overactive bladder, interstitial cystitis, bladder diverticula and urothelial carcinoma or those persistently receiving opioid were also excluded.
Procedures
All Patients who attended the pelvic pain unit for the first time were evaluated using the UPOINT phenotype classification system by a fellowship trained urologist. They were subjected to detailed history and physical examination including comprehensive digital rectal examination looking for prostate size, consistency, tenderness, and palpable abnormalities in addition to thorough assessment of pubococcygeus and iliococcygeus muscles. Patients diagnosed with pelvic floor muscle tenderness were referred for the PEMM therapy, which was provided by physiotherapists under the direction of a qualified physiotherapy specialist with a PhD in myofascial therapy.
The PEMM Therapy
The PEMM therapy for CPPS mobilizes the fascia surrounding the lumbopelvic area through the myofascial connectivity of the trunk referred to as the oblique slings [25-28] (Figure 1). The anterior oblique sling (AOS) is made up of the external oblique (EO) and internal oblique (IO) muscles, which connect to the contralateral hip adductor (HA) muscles via the adductorabdominal fascia (AAF) [26]. The Posterior Oblique Sling (POS), also known as the back functional line, is made up of the latissimus dorsi (LD) and the contralateral gluteus maximus (GMx) joined by thoracolumbar fascia (TLF) [29]. Following the completion of the initial evaluation and probable exclusion based on contraindications, patients eligible for the PEMM therapy were given four sessions of therapy (S1, S2, S3, and S4) once a week, with each session lasting an average of 35 minutes. The PEMM therapy involves ‘hands-on’ mobilization of fascial restrictions in the designated areas of dysfunction [20]. The four sessions were designed to mobilize the chosen PEMM locations on the oblique slings with the aim of releasing soft tissue constraints and pressure around the pelvis [20]. The NRS pain scale and its application were explained to the patients before the session. The PEMM therapy session can be grossly divided into posterior sessions (S1 & S2) & anterior sessions (S3 & S4).
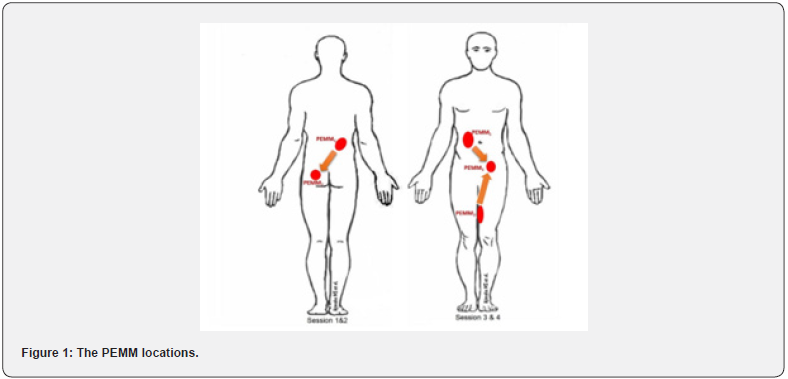
Posterior PEMM sessions (S1 & S2): For the posterior PEMM sessions (S1 & S2), the diagonal connectivity of ipsilateral LD and TLF with contralateral GMx were attended through intense mobilizing sessions. Patients were comfortably placed in prone position with hands by their sides and a pillow under the abdomen. For locating PEMM1 point, an area at the level of the L1 vertebral spinous process in the mid-clavicular line was identified. A scanning palpation was used in this area to look for any myofascial restrictions or tenderness. Then, a point of maximum tenderness was identified with its corresponding NRS score. While palpating, any pain pattern or radiating pain reproduction was recorded. The area was prepared for PEMM therapy once the pain was verbalized by the patient.
To standardize the treatment, a 7-minute mobilization session was adopted for all the PEMM points [30]. Two PEMMtrained physiotherapists mobilized the PEMM points in a depthincreasing manner for 7 minutes using reinforced fingers and elbows. The two-therapist arrangement was used to prevent the PEMM therapists from becoming physically exhausted. The end point’s NRS on palpatory tenderness was recorded when the allotted time had elapsed. The patient was then allowed to stay for 5 minutes with relaxed diaphragmatic breathing. The second point (PEMM2) on the contralateral GMx was then identified for therapy. The PEMM2 point was palpated by first locating the posterior superior iliac spine (PSIS). The midpoint of the bilateral PSIS was determined, and a location one inch below it was marked. From this point, an imaginary line was drawn to the greater trochanter. The midpoint of this line served as the PEMM2’s initial point of contact. PEMM2’s greatest point of tenderness and the NRS scores were recorded. After the session, patients were given a week of rest with an education on possible adverse events and lifestyle instructions.
Anterior PEMM sessions (S3 & S4): The AOS was targeted in the third and fourth PEMM therapy sessions (S3 &S4). The PEMM points were identified on the ipsilateral EO, contralateral AAF and HA muscles. Mobilizing three PEMM points for each of the left and right AOS was the focus of the S3 and S4 sessions. Patients were positioned in supine lying with hands by the side and a pillow under the knee for the PEMM1 on the ipsilateral EO. For palpation, a point, two inches lateral to the umbilicus was chosen. A scanning palpation was performed at this point to locate any tenderness or myofascial restriction. The point for the therapy was marked after recording the NRS for the highest palpatory tenderness. On PEMM1, a 7-minute myofascial mobilization was performed with a post mobilization NRS recording. The mobilization of the PEMM2 point was initiated at the contralateral hip adductor region following a 5-minute rest period. Palpation for myofascial restrictions was performed at the intersection of the upper 2/3 and lower 1/3 of the medial thigh. The third point, PEMM3, was palpated at the hip joint on the side of the PEMM 2 point in the adductor-abdominal fascia (AAF). The patients were asked to actively flex their hips with knee extended to identify the iliopsoas tendons. A point, one inch medial to it was then located for the PEMM3 mobilization. Due to the close vicinity of the neurovascular structures, this area was treated with caution. The S4 PEMM points were the same as the S3 sessions, but on the opposite side. All patients were advised to resume their activities and work the day following the session.
Data Collection
Patients’ demographic and clinical data were gathered for analysis. These included age, duration of symptoms, associated symptoms (pain, urinary symptoms, sexual dysfunction) and UPOINT phenotypes. Symptom severity was assessed using the National Institute of Health-Chronic Prostatitis Symptom Index (NIH-CPSI) and the numerical rating scale (NRS). These scales were utilized at 3 time points to evaluate treatment response: baseline (time 0), 1 week after the fourth session (time 1) and 9 months following treatment (time 2). The NIH-CPSI comprises three subscales that measure pain (score range 0-21), urinary symptoms (score range 0-10), and quality of life (QoL) (score range 0-12), with a total score range of 0 to 43 [31]. Higher scores indicate more severe symptoms. A clinically relevant improvement of symptoms is defined as a 6-point drop in the CPSI score ([31]). Patients were classified according to the NIH-CPSI results into mild (0-15), moderate (16-29) or severe (>29) groups of symptoms severity [32]. By asking patients to rate their mean pain intensity for the past 24 hours, the NRS scale was utilized to track their subjective and general pain complains.
Results
A total of 29 subjects met the inclusion criteria and their data was used for statistical analysis. Table 1 displays baseline clinical data and patient demographics. The mean (± SD) patient age was 41.21 (± 8.69) years while the average symptom duration was 4 years. All patients reported pelvic pain and tenderness. Almost half of the patients had associated urinary symptoms while a third of them complained of sexual dysfunction (Figure 2).
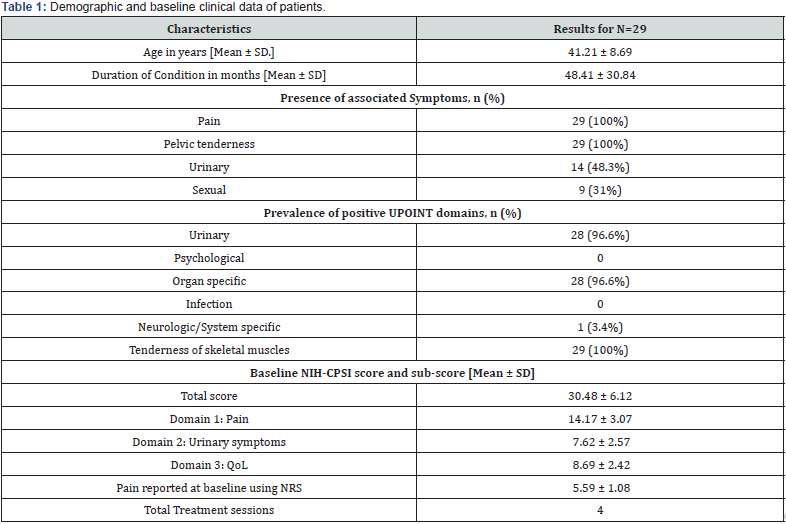
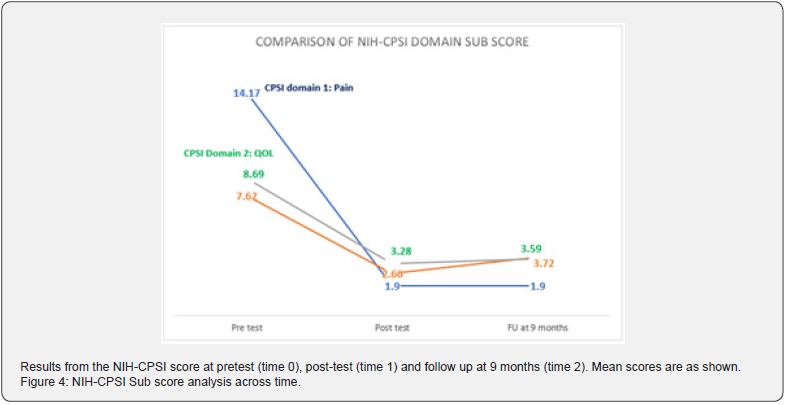
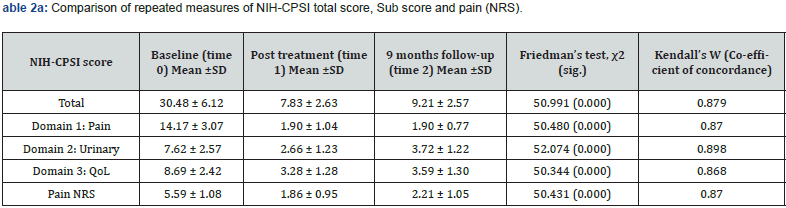
As all patients were specifically referred for the PEMM therapy, the muscle tenderness UPOINT phenotype was prevalent in all of them. Concomitant organ confined and urinary domains were reported in the majority of patients (96.6%, for both). Only one patient had an associated neurogenic phenotype. Multiple domains were identified in all patients. The average total NIHCPSI score before treatment was 30.48 ± 6.12 ranging from 13 to 37. Prior to treatment, the mean (± SD) NIH-CPSI sub scores for pain, urinary symptoms, and quality of life were 14.17 (± 3.07), 7.62 (± 2.57), and 8.69 (± 2.42), respectively. The baseline NRS score for the 24-hour pain behavior was 5.59 (± 1.08) (Table 1).
The efficacy of the PEMM therapy was evaluated though the comparison of changes in NIH-CPSI and NRS scores at the three time points (time 0, time 1 and time 2) (Table 2a). The PEMM therapy resulted in significant symptom reduction in 96.6% of the individuals studied, as indicated by a minimum 6-point change in their CPSI score. The significant reductions in NIH-CPSI and NRS scores seen at time 1 (74.3% and 66.7%) were relatively retained at time 2 (69.8% and 60.5%, respectively) compared to time 0 (Figure 3 &4). While an increase in total NIH-CPSI and NRS scores were noted between time 1 and time 2, results observed at time 2 were lower than those obtained at the baseline (time 0). More than 95% of patients reported a reduction in pelvic symptoms of at least 50% following the final session of the PEMM therapy.
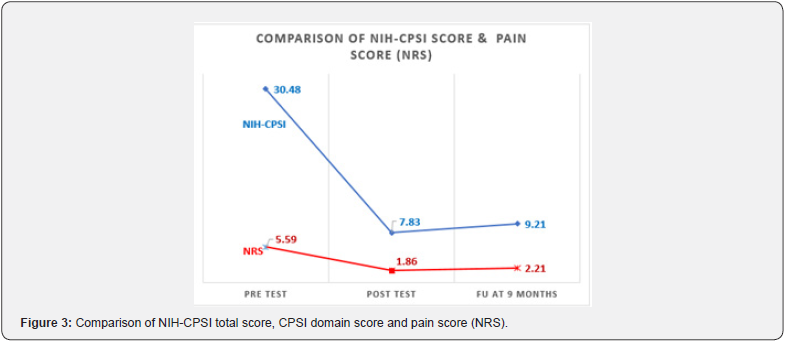
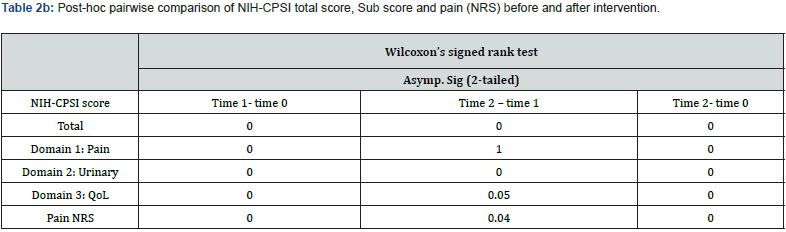
There existed a significant difference (p<.05, CI 95%) in NRS score and NIH-CPSI total score as well as sub-scores immediately after the intervention (time 1) and also at time 2 as demonstrated by the Friedman Chi-square value (Table 2a). Mean scores were lower at time 1 compared to time 2. However, post-hoc pairwise comparison (Table 2b) demonstrated a negative change in NIHCPSI total score and sub-score for urinary symptoms when time 2 was compared to time 1. NIH CPSI sub-score for pain had remained the same at time 2, and so does the QoL sub-score as well as the NRS pain assessment which showed no further deterioration at time 2 in comparison with time 1 (Figure 3 & 4). Most of the improvements were reported at the time 1, were relatively retained until time 2 except for the urinary sub-score.
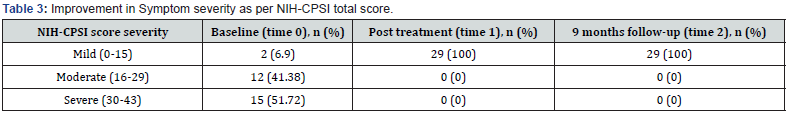
At baseline, about half of the patients (51.72%) reportedly had severe symptoms, while more than a third (41.8%) had moderate symptoms and only 2 subjects (6.9%) had mild symptom severity. No subjects belonged to the moderate or severe symptom groups both at times 1 and 2 following treatment (Table 3). Pain, feverishness, dysuria, and skin discoloration in the area of PEMM application were among the side effects reported during the therapy. All of the adverse effects seen were transient, and none of the patients reported any adverse effects or its retention after the fourth session of therapy.
Discussion
A comprehensive four session PEMM therapy focused on myofascial connectivity reduced the CPPS symptoms immediately following the therapy and remained largely unchanged for 9 months when measured with the NIH-CPSI and NRS scales. Immediately following the PEMM therapy, the NIH-CPSI score decreased by 74.3 % and it remained at 69.8% at the follow up, demonstrating a strong short- and long-term response posttherapy. Similar pain relief results have been observed in soft tissue-biofeedback-cognitive therapy-based studies that have shown superior symptom reductions [20,24,32-35]. The PEMM therapy has an advantage over the therapies used in these studies in that the application’s root is “external” and therapy requires a few sessions (four) only to produce the desired outcome. No participants reported a worsening of their symptoms post-test. When compared to baseline scores, more than 95% of patients’ pelvic symptoms were reduced by 50% or less at the posttest and follow-up. We found the treatment to be safe, with only minor and temporary side effects. This suggests that the PEMM therapy is a safe and feasible adjuvant for the treatment of males with predominantly tenderness positive CPPS and is unlikely to cause harm or exacerbate symptoms. This study backs up the conclusions of a prior retrospective study on the effects of external myofascial mobilization on chronic pelvic pain in males [20].
Because of the broad spectrum of symptoms, CPPS continues to be a mystery for medical professionals and patients [34]. Medication or psychotherapy alone is frequently ineffectual and can frustrate both the patient and the physician [34]. The primary treatment strategy for the majority of practitioners is medical therapy focused on specific symptoms. Antibiotics are regularly recommended, even when there is no sign of infection. Frequently used pharmacotherapies including anti-inflammatories, α-blockers and anticholinergics, may be beneficial in some contexts [36]. The results of a 2018 meta-analysis [33] and 2019 systematic reviews [24,37] evaluating the efficacy of non-medical approaches for CP/CPPS, showed that treating CPPS with nonpharmacological therapies, including myofascial techniques, can improve the outcomes.
Despite the high prevalence of CPPS, the myofascial component has not received adequate attention or care, as per the European Association of Urology Guidelines Panel [22]. The panel concluded that the treatment of the myofascial pain component was important for the overall outcome of treatment and that myofascial pain needed to be assessed in all patients with CPPS [22]. Men with CPPS frequently experience pelvic floor tension and pain and addressing this issue can significantly reduce patients’ symptoms [17,20,28]. The majority of studies that have been done thus far on the myofascial concept have used a variety of trigger point releases in order to reduce the muscular tension and discomforts [21,33,38]. Although trigger point therapy can provide rapid symptom relief, if the underlying myofascial dysfunction is not addressed, the impact is often temporary.
Significant improvements in the NIH-CPSI score and pain by visual analogue scale were reported after myofasciabased therapy in studies with refractory CPPS [21,34]. Similar outcomes were also observed immediately following therapy in a retrospective trial that included external myofascial mobilization [20]. The current study made use of a rationale based on evidence that took into account various myofascial properties including its connectivity [18], force transmission [19], and its role in the emergence of pelvic dysfunction and pain (Horton, 1992).
Studies have shown that men with CP/CPPS exhibited increased PFM resting activity, even while they were at rest, pointing to a compromised central nervous system’s capacity to relax the PFM [5]. Men with CP/CPPS have also been found to have higher sympathetic drive [5,39]. Daily movements and faulty positions that activate MTrPs prevent muscles from fully relaxing, limiting its flexibility [22]. Trigger point activation through somatovisceral and viscerosomatic interaction this way gets amplified and sustained. This, in conjunction with other mechanisms, results in the peripheral and central nervous system sensitization [40].
The PEMM therapy for CPPS mobilizes the fascia around the lumbopelvic area through the trunk’s oblique myofascial connectivity (Figure 1) called anterior and posterior oblique slings [25-28]. Sling systems work to create force vectors that help with intra- and inter-myofascial force transfer between the lumbopelvic area [27]. This system’s dysfunction hinders performance in terms of strength, speed, and power [29]. With the exception of a few studies, there hasn’t been much research on the CPPS and the role of myofascial sling connectivity [20,27]. Pelvic pain and dysfunction can be caused by myofascial restrictions in the oblique connectivity that interfere with muscle contraction and stiffness in the soft tissues of the lumbo-pelvic region [27,41]. It is possible that the positive results seen in this study are the result of changes in the length and stiffness of the pelvic soft tissues in response to the biomechanical remodeling of the myofascial slings brought on by the PEMM therapy. The neurological drive to the muscles, which is mediated by muscle afferents and spinal inhibitory reflexes, has been reported to be significantly reduced by manual treatments focused on soft tissue stretching [5]. So, it is also likely that increasing neuromuscular control of the anterior and posterior oblique systems, in conjunction with the biomechanical correction, contributed to a reduction in symptoms via enhancing pelvic girdle stability. Further scientific research is needed to verify these claims, though.
To the best of our knowledge, the present study is the first of its kind that assessed the short- and long-term efficacy of a comprehensive four- session PEMM therapy program. We used a closed protocol for all patients and all sessions. As shown, the PEMM approach led to a significant reduction in the NIHCPSI score (74.3%) and NRS score (66.2%) post treatment with retention of benefit up to 69.8% in the CPSI score and 60.5% in the NRS score at FU compared to the baseline, signifying a robust response. There existed a significant difference (p<.05, CI 95%) in NRS score and NIH-CPSI total score as well as sub-scores immediately after the intervention and also at the FU. Mean scores were lower immediately post treatment as compared to FU. Item analysis of CPSI revealed that the pain domain demonstrated the most significant reduction, 86.59% at the post test, which was retained up to nine months.
The QoL demonstrated a significant change with almost 62% reduction of symptoms with a retention rate of 59% at the FU. The urinary domain showed an improvement of 65% at the posttest but the effects started diminishing at the FU. This finding was justified by the post-hoc pairwise comparison of the domains (Table 2b). NIH-CPSI sub-score for pain had remained the same at the FU, and so does the QoL sub-score. One can’t argue for difference in the effect of PEMM therapy on the sub domains of CPSI scale based on the finding from a retrospective study, but the item analysis is pointing towards a higher therapeutic effect of the PEMM on pain domains and QOL domains compared to the urinary symptoms of CPPS. The retention effect of the QOL domain infers that the QoL is more related to the pain domain than the urinary symptom domain. Future prospective controlled studies can give more detailed and specific answers to the above findings.
The present study had the advantages of including patient data with a “predominantly tenderness positive phenotype,” a reasonably young patient population, and adherence to a closed therapy protocol. All patients received similar therapy from the same physiotherapists for a set duration and frequency. Our study’s major limitation was its retrospective format, which prevented us from having control groups or conducting randomized controlled trials. The non-comparative single cohort model was selected as the optimum methodology since it would not go against the ethical standards of treatment for patients who are seeking relief from their crippling pain. The adoption of a placebo control group would violate our patients’ trust since we would purposefully not treat control group patients who also needed relief.
The results may be subject to a super realization bias as a result of the small sample size, the relatively shorter duration of the condition, and the stringent inclusion criteria. The early administration of the PEMM therapy, regardless of the medical therapy obtained, may have influenced the outcome. The retrospective design of the study makes it difficult to control the potential confounders that could affect the results and the placebo effects. There is a significant likelihood that the therapeutic effects we saw in this study will wane with time due to the complicated pathomechanism of chronic pain. Future plans may involve applying the PROMIS-29 outcome measure to evaluate the physical and mental well-being of our patients in order to more fully examine the clinical significance of our findings.
Conclusion
A comprehensive, closed protocol PEMM therapy based on the myofascial sling connectivity led to a significant improvement in symptoms in all the patients studied. The relative retention of effects found in the pain and the quality-of-life domains at the follow-up was particularly promising. The PEMM therapy may be a useful treatment adjunct for CPPS that is primarily tenderness positive. To confirm the aforementioned findings and to identify CPPS phenotypes that might respond better to such therapies, future prospective, randomized controlled studies with a sufficient sample size and longer follow-up should be implemented.
CRediT Authorship Contribution Statement
Ajimsha MS: idea, Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Validation Writing - original draft. Ahmad Majzoub: Methodology, Data curation, Formal analysis, Writing - review & editing. Pramod D. Shenoy: Methodology, Formal analysis, Validation, Visualization, Writing - review & editing. Laith Ahmad Ismail: Investigation, Project administration, Supervision, Visualization. Smithesh Kooven: Project administration, Resources, Software, Validation, Writing - review & editing
Acknowledgement
We gratefully acknowledge Ms. Noora Al-Mudahka, AED and Chief of Physiotherapy, for her wholehearted support and invaluable contributions to this study.
Funding
This research received no external funding. The open access publishing of this study was supported by the Qatar National Library.
References
- Smith CP (2016) Male chronic pelvic pain: An update. Indian J Urol 32(1): 34-39.
- Shoskes DA, Nickel JC (2013) Classification and treatment of men with chronic prostatitis/chronic pelvic pain syndrome using the UPOINT system. World J Urol 31(4): 755-760.
- Collins MM, Stafford RS, O’Leary MP, Barry MJ (1998) How common is prostatitis? A national survey of physician visits. J Urol 159(4): 1224-1228.
- Krieger JN, Lee SW, Jeon J, Cheah PY, Liong, ML, et al. (2008) Epidemiology of prostatitis. Int J Antimicrobial Agents 31(Suppl 1): S85-S90.
- Yani MS, Eckel SP, Kirages DJ, Rodriguez LV, Corcos DM, et al. (2022) Impaired ability to relax pelvic floor muscles in men with chronic prostatitis/chronic pelvic pain syndrome. Phys Therap 102(7): pzac059.
- Armour M, Lawson K, Wood A, Smith CA, Abbott J (2019) The cost of illness and economic burden of endometriosis and chronic pelvic pain in Australia: A national online survey. Plos One 14(10): e0223316.
- Krieger JN, Nyberg L, Nickel JC (1999) NIH consensus definition and classification of prostatitis. JAMA 282(3): 236-237.
- Engeler DS, Baranowski AP, Dinis-Oliveira P, Elneil S, Hughes J, Messelink EJ, (2013) The 2013 EAU guidelines on chronic pelvic pain: Is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. Eur Urol 64(3): 431-439.
- Crofts M, Ramsden S, Horner P (2022) Chronic pelvic pain in men. Med 50(5): 280-284.
- Anderson RU, Sawyer T, Wise D, Morey A, Nathanson BH (2009) Painful myofascial trigger points and pain sites in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol 182(6): 2753-2758.
- Fitzgerald MP, Anderson RU, Potts J, Payne CK, Peters KM, et al. (2013) Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J Urol 189(Suppl 1): S75-S85.
- Giubilei G, Mondaini N, Minervini A, Saieva C, Lapini A, et al. (2007) Physical activity of men with chronic prostatitis/chronic pelvic pain syndrome not satisfied with conventional treatments-Could it represent a valid option? The physical activity and male pelvic pain trial: A double-blind, randomized study. J Urol 177(1): 159-165.
- Chiarioni G, Nardo A, Vantini I, Romito A, Whitehead WE (2010) Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterol 138(4): 1321-1329.
- Kessler TM, Mordasini L, Weisstanner C, Jüni P, da Costa BR, et al. (2014) Sono-electro-magnetic therapy for treating chronic pelvic pain syndrome in men: A randomized, placebo-controlled, double-blind trial. Plos One 9(12): e113368.
- Sakr AM, Fawzi AM, Kamel M, Ali MM (2022) Outcomes and clinical predictors of extracorporeal shock wave therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: A prospective randomized double-blind placebo-controlled clinical trial. Prostate Cancer Prostat Dis 25(1): 93-99.
- Marinkovic SP, Gillen LM, Marinkovic CM (2011) Minimum 6-year outcomes for interstitial cystitis treated with sacral neuromodulation. Int Urogynecol J 22(4): 407-412.
- Anderson RU, Wise D, Sawyer T, Chan C (2005) Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J Urol 174(1): 155-160.
- Ajimsha MS, Shenoy PD, Gampawar N (2020) Role of fascial connectivity in musculoskeletal dysfunctions: A narrative review. J Bodywork and Movement Therap 24(4): 423-431.
- Ajimsha MS, Shenoy PD, Surendran PJ, Jacob P, Bilal MJ (2022) Evidence of in-vivo myofascial force transfer in humans- a systematic scoping review. Journal of Bodywork and Movement Therapies 32: 183-195.
- Ajimsha MS, Ismail LA, Al-Mudahka N, Majzoub A (2021) Effectiveness of external myofascial mobilization in the management of male chronic pelvic pain of muscle spastic type: A retrospective study. Arab J Urol 19(3): 394-400.
- Anderson R, Wise D, Sawyer T, Nathanson BH (2011) Safety and effectiveness of an internal pelvic myofascial trigger point wand for urologic chronic pelvic pain syndrome. Clin J Pain 27(9): 764-768.
- Abreu-Mendes P, Baranowski AP, Berghmans B, Borovicka J, Cottrell AM, et al. (2023) Myofascial Pelvic Pain: Best Orientation and Clinical Practice. Position of the European Association of Urology Guidelines Panel on Chronic Pelvic Pain. Eur Urol Focus 9(1): 172-177.
- Oyama IA, Rejba A, Lukban JC, Fletcher E, Kellogg-Spadt S, et al. (2004) Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urol 64(5): 862-865.
- Klotz SGR, Schön M, Ketels G, Löwe B, Brünahl CA (2019) Physiotherapy management of patients with chronic pelvic pain (CPP): A systematic review. Physiotherap Theo Practice 35(6): 516-532.
- Dorman TA (1992) Storage and release of elastic energy in the pelvis: Dysfunction, diagnosis and treatment. J Ortho Med 14(2): 54-62.
- Brown SH, McGill SM (2009) Transmission of muscularly generated force and stiffness between layers of the rat abdominal wall. Spine 34(2): E70-E75.
- Lee DG (2011) The pelvic girdle: An integration of clinical expertise and research. Elsevier Health Sciences.
- Horton RC (2015) The anatomy, biological plausibility and efficacy of visceral mobilization in the treatment of pelvic floor dysfunction. Journal of Pelvic, Obstet Gynaecol Physiotherap 117: 5-18.
- Lim W (2021) Tensile force transmission from the upper trunk to the contralateral lower leg throughout the posterior oblique sling system. Int J Human Movement and Sports Sci 9(2): 294-300.
- Fonta M, Tsepis E, Fousekis K, Mandalidis D (2021) Acute effects of static self-stretching exercises and foam roller self-massaging on the trunk range of motions and strength of the trunk extensors. Sports 9(12): 159.
- Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Dunn RL, et al. (2009) Rescoring the NIH chronic prostatitis symptom index: Nothing new. Prostate Cancer & Prostatic Dis 12(3): 285-287.
- Arda E, Cakiroglu B, Tas T, Ekici S, Uyanik BS (2016) Use of the UPOINT classification in Turkish chronic prostatitis or chronic pelvic pain syndrome patients. Urol 97: 227-231.
- Anderson RU, Wise D, Nathanson BH (2018) Chronic prostatitis and/or chronic pelvic pain as a Psycho neuromuscular disorder-A Meta-analysis. Urol 120: 23-29.
- Masterson TA, Masterson JM, Azzinaro J, Manderson L, Swain S, et al. (2017) Comprehensive pelvic floor physical therapy program for men with idiopathic chronic pelvic pain syndrome: A prospective study. Translat Androl Urol 6(5): 910-915.
- Polackwich AS, Li J, Shoskes DA (2015) Patients with pelvic floor muscle spasm have a superior response to pelvic floor physical therapy at specialized centers. J Urol 194(4): 1002-1006.
- Tadros NN, Shah AB, Shoskes DA (2017) Utility of trigger point injection as an adjunct to physical therapy in men with chronic prostatitis/chronic pelvic pain syndrome. Translat Androl and Urol 6(3): 534-537.
- Fuentes-Márquez P, Cabrera-Martos I, Valenza MC (2019) Physiotherapy interventions for patients with chronic pelvic pain: A systematic review of the literature. Physiother Theory Pract 35(12): 1131-1138.
- Grinberg K, Weissman-Fogel I, Lowenstein L, Abramov L, Granot M (2019) How does myofascial physical therapy attenuate pain in chronic pelvic pain syndrome? Pain Res and Manage 2019: 6091257.
- Yilmaz U, Liu YW, Berger RE, Yang CC (2007) Autonomic nervous system changes in men with chronic pelvic pain syndrome. J Urol 177(6): 2170-2174.
- Travell JG, Simons DG (1992) Myofascial pain and dysfunction: The trigger point manual. Lippincott Williams & Wilkins.
- Hoffman J, Gabel P (2013) Expanding Panjabi’s stability model to express movement: A theoretical model. Medical Hypotheses 80(6): 692-697.






























