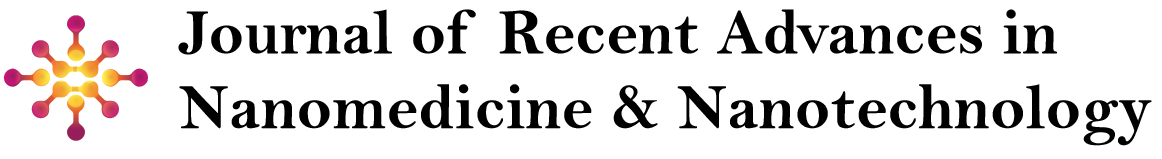Abstract
Chemotherapy is an important treatment for breast cancer, in order to solve the multiple toxic side effects of breast cancer chemotherapy on patients, nanoparticle drug carrier came into being. Currently available Fe3O4/carbon-based composites have the following deficiencies such as complex preparation procedures and poor stability. The aim of this study was to synthesize a ZIF-8-modified GO@Fe3O4 nanoparticle drug carrier, the carrier has the advantages of simple preparation and high stability. Subsequently, this study proved that the vector had good biocompatibility and very low cytotoxicity through cytotoxicity and flow cytometry, GO-Fe3O4-ZIF-8 has potential application prospects as a better carrier of chemotherapy drugs for breast cancer.
Keywords: Breast cancer; Nanocarriers; Fe3O4; Graphene oxide; ZIF-8; Hydrochloride; Graphene oxide; Potassium permanganate; Carbon dioxide; Mitotic period; Hydrogen Peroxide; Potassium permanganate; Hyaluronic acid; Ferric chloride; Potential of hydrogen
Abbreviations: ZIF-8: Zeolite imidazolate-frame-8; SIM: Simvastatin; CEL: Celastrol; GO: Graphene oxide; Fe3O4: Ferroferric oxide; HA: Hyaluronic acid; AS: Atherosclerosis; PH: Potential of hydrogen; ZnO: Zincoxide; NaNO3:Sodium Nitrate ; KMnO4: Potassium permanganate; H2O2: Hydrogen Peroxide; HCL: Hydrochloric acid; FeCl2: Ferrous chloride; FeCl3: Ferric chloride; Fe2+: Ferrous iron; Fe3+: Ferri ion; N2; Nitrogen; PEI: Polyetherimide; DMSO: Dimethyl sulfoxide; EDC: Hydrochloride; NO3: NITRATE; Zn: Zinc; H2O: Water; CO2: Carbon dioxide; MEM: Minimum Essential Medium; DMEM: Dulbecco’s modified eagle medium; PBS: Phosphate Buffered Saline; CCK-8: Cell Counting Kit-8; OD: Absorbance; SEM: Scanning Electron Microscope; G0: Gap phase0; G1: Gap phase1; S: Synthesis phase; G2: Gap phase2; M: Mitotic period
Introduction
The latest statistics for 2024 show that breast cancer is the most common malignancy in women, for women, breast cancer, lung cancer, and colorectal cancer accounted for 51 percent of all newly diagnosed malignancies, with breast cancer alone accounting for 32 percent. And since the mid-2000s, breast cancer rates in women have slowly increased by about 0.6 percent per year [1]. Chemotherapy, targeted therapy, endocrine drug therapy, radiotherapy and so on are still commonly used treatment methods [2]. Systemic chemotherapy is still an effective and important treatment for breast cancer; however, the drug effects of systemic chemotherapy do not only affect breast cancer cells, its impact on other systems of the human body cannot be ignored. For example, for long-term tamoxifen treatment lasting more than 2 years, 12% of patients will have retinal changes [3] and irreversible after withdrawal, which has a great impact on patients’ life treatment; Cardiovascular toxicity from chemotherapy drugs is associated with clinically relevant adverse outcomes, and cardiovascular disease is the leading cause of death among breast cancer survivors, accounting for 15.9% of deaths, followed by breast cancer at 15.1% [4]. Platinum drugs often cause kidney damage and renal failure. In order to better prognostic outcome and subsequent quality of life of clinically cured patients, nanometer drug-carrying materials are developing rapidly in recent years. The existing magnetic nanodrug carrier materials are one of the carrier materials with the potential to achieve local targeting and release. Coupling Fe3O4 with carbon-based materials can improve its performance as magnetic drug carriers. However, the preparation procedure of Fe3O4/carbon-based composites as magnetic drug carriers reported at present is complicated [5] and has poor stability.
Compared with traditional magnetic nanodrug carrier materials, graphene oxide nanoparticles have received more attention in tissue engineering due to their two-dimensional planar structure, chemical stability, excellent photosensitivity, excellent electrical conductivity, high surface area, excellent mechanical strength and stiffness [6]. At this stage, graphene oxide is mostly used for sewage treatment [7,8] and adsorption of chemical substances [9]. And studies have proved that it is renewable and recyclable as an adsorbent, and will not significantly reduce performance [10]. At present, graphene oxide has been preliminarily used in cancer cell imaging, chemotherapy drug targeting, chemotherapy, photothermal therapy and photodynamic therapy [11]. At present, graphene oxide has been preliminarily used in cancer cell imaging, chemotherapy drug targeting, chemotherapy, photothermal therapy and photodynamic therapy [11]. Many compounds have been functionalized on the GO surface for biological applications and to minimize cytotoxicity. In order to further enhance the stability, efficiency and drug loading stability of the composite, imidazolate-frame-8(ZIF-8) was modified on GO@Fe3O4 in this study. Studies have shown that the adsorption efficiency of lead by graphene oxide can be improved under the modification of ZIF-8 [12], and it shows good synergistic efficiency with the original material. ZIF-8 is a nanocarrier modification component that has received more and more attention in recent years. It can enhance the stability of carrier materials and is a good ph responsive drug delivery vector [13]. Celastrol (CEL) has been encapsulated in zeolite ZIF-8 nanoparticles for the treatment of ovarian cancer, showing favorable properties including excellent water solubility, high drug loading (31.60%±2.85), encapsulation efficiency (60.52%±2.79), and minimal side effects [14]. In other studies, celecoxib was loaded into ZIF-8 named CEL@ZIF-8 for the treatment of chronic osteomyelitis, and the ion release and drug release experiments showed that the drug carrier responded well to pH value and could control the release of ions and drugs, and the drug release system had multiple functions such as antibacterial, osteogenic, anti-inflammatory and intelligent release [15]. In other animal experiments, simvastatin (SIM) was effectively encapsulated in zeolite imidazolate frame-8 to construct SIM/ZIF- 8@HA for the treatment of atherosclerosis. It was found that SIM/ ZIF-8@HA could release drugs at the target site and effectively inhibit the development of AS plaques, without any obvious side effect1 in the treatment of mice [16]. In conclusion, ZIF-8 is an excellent modification material, which can enhance the stability of drug carrier, achieve more accurate release of therapeutic drugs in target cells, and reduce the toxic side effects of drugs on human body. Therefore, we consider modifying ZIF-8 onto GO@Fe3O4 nanocarriers to increase stability and reduce toxic side effects.
At present, the drug carrier used in clinical treatment should have good biocompatibility. We confirmed the biosafety of ZIF-8-modified GO@Fe3O4 nanocarriers by cytotoxicity and flow cytometry. At present, it has been reported that similar composite photocatalysts ZnO/3O4-GO/ZIF have been used to degrade pharmaceutical contaminants, and it has been found that it has better adsorption catalytic capacity and can support multiple recycling [17]. Based on the current relevant studies, it is not difficult to find that the composition and similarity of the carrier are mostly used to solve the purification of polluted water sources and the adsorption of chemical substances, and it has been confirmed that the biosafety is good, and the combination of graphene oxide can be considered as a drug carrier[18], our constructed ZIF-8-modified GO@Fe3O4 nanocarriers may be better carriers for breast cancer chemotherapeutics.
Materials and Testing
Preparation of graphene oxide (GO)
Nanoscale graphene oxide (GO) was fabricated by the modified Hummers [19] method. The detailed operation is as follows: Under the condition of 4°C, add 1 g of graphite powder and 0.5 g of NaNO3 into 23 ml of concentrated sulfuric acid in sequence, and stir for a reaction lasting for 1 hour. Upon completion, 3 g of KMnO4 was added at 10°C and the mixture was stirred for a reaction lasting 2 hours. The reaction was continued with stirring in a 38°C water bath for 0.5 hour. Subsequently, 80 ml of deionized water was slowly added, and the mixture was stirred for a reaction at 95°C for 0.5 hour. Finally, 15 ml of 30% H2O2 and 37 ml of deionized water were added, and the mixture was stirred for a reaction of 0.5 hour. Once the reaction was finished, hot suction filtration was carried out, followed by washing with a 10% HCL solution and then with deionized water until the filtrate became neutral. After adding deionized water for dispersion and subjecting it to ultrasound for approximately 1 hour, an aqueous solution of graphene oxide was obtained. Solid graphene oxide could be obtained through freeze-drying.
Preparation of Fe3O4
Nanoscale Fe3O4 was fabricated through the chemical coprecipitation approach [20]. First of all, 50 ml of deionized water and a mixture of 5.1 mg of FeCl2·4H2O and 13.9 mg of FeCl3·6H2O (with the chemical reaction ratio of Fe2+:Fe3+ being 1:2) were vigorously stirred at room temperature under N2 protection. Subsequently, the temperature was elevated to 45°C, and ammonia water was added dropwise until the reaction system was in an environment with a pH ranging from 9 to 10. Stirring was continued for 15 minutes. Once the reaction was accomplished, the mixture was subjected to heat preservation and aging at 75°C for 15 minutes. Finally, the reaction liquid was cooled to room temperature and washed three times with an ethanol aqueous solution (1:1) to obtain colloidal Fe3O4 Solid Fe3O4 could be obtained through vacuum drying at 40°C.
Preparation of GO@Fe3O4
0.65g of FeCl3-6H2O was dissolved in 20mL of ethanol, to which 0.2g of trisodium citrate salt was added and stirred to dissolve. During the stirring process, 1.2g of sodium acetate was added slowly and stirred vigorously for 30 min. the reaction solution was taken out and poured into a 50mL reactor and heated in a blast drying oven at 200°C for 10 h. The reaction solution was taken out, washed alternately with ethanol and deionized water, and dried in a vacuum drying oven at 60°C overnight to obtain solid Fe3O4 nanoparticles.
Presentation of GO@Fe3O4-PEI
0.3 g of polyetherimide (PEI) was dissolved in distilled water, and an appropriate amount of DMSO was added to facilitate dissolution. Meanwhile, 0.2 g of GO@Fe3O4 was fully dispersed in distilled water, and 0.1g of 1-(3-dimethylaminopropyl)-3- ethylcarbodiimide hydrochloride (EDC) was added for activation, followed by stirring for 3 hours. Subsequently, under stirring conditions, PEI was added dropwise to the activated GO@Fe3O4, and the mixture was stirred at room temperature for 24 hours. Centrifugal washing was carried out to remove the unreacted PEI, obtaining GO@Fe3O4-PEI.
Presentation of ZIF-8-GO-Fe3O4
0.24g of 2-methylimidazole, 0.05g of polyvinylpyrrolidone and 0.02g of Fe3O4 nanoparticles were ultrasonically dispersed in 10mL deionized water, denoted as solution 1. 0.0928 g of Zn (NO3)2·6H2O and 0.2g of GO were ultrasonically dispersed in 10mL tert-butanol, denoted as solution 2. The solution 1 and solution 2 were mixed, with the magnetic force stirred evenly, then the stirred solvent was centrifuged in a centrifuge at 12000 rpm for 5min and the bottom layer was washed with anhydric ethanol. Finally, the ZIF-8-GO-Fe3O4 was obtained by drying the mixture overnight in a vacuum drying oven at 60℃ [21].
Cytotoxicity Experiment
Normal mammary cells, normal renal tubular epithelial cells and normal gastric mucosa (MCF-10A, HK-2, L-O2) cells were respectively cultured in MCF-10A-specific medium, MEM medium and DMEM medium containing 10% fetal bovine serum. Cells in the logarithmic growth phase and in good growth condition were taken and seeded in 96-well plates at a density of 5*103 cells/well, with a total of 100 μl of cell suspension added to each well. The cells were cultured overnight at 37°C and 5% CO2. The cells were grouped according to the addition of GO-Fe3O4-ZIF-8 drug-loaded particles of different concentrations (0, 10, 50, 100, 150, 200, 250 μg/ml), and three replicate wells were set for each group, and the experiment was repeated three times. The treatment method of the well plate was as follows: The original medium was aspirated, gently washed three times with PBS, and the complete medium containing the corresponding carrier concentration was replaced. After 48 hours, 10 μl of CCK-8 reagent was added to each well and shaken well for uniform mixing. The 96-well plate was placed in the incubator for incubation for 2 hours. The absorbance (OD) at 450 nm was measured by a microplate reader.
Flow cytometry cell cycle experiment
Normal cells of different tissues were seeded in 6-well plates. When the cell confluence reached 40%-50%, the GO-Fe3O4-ZIF-8 drug carrier with a concentration of 250 μg/ml was added and cultured for 48 hours. Prepare 70% ethanol in advance and precool it, and also precool PBS. Digest and collect the cells in the 6-well plates with trypsin, centrifuge at 1000 rpm for 5 minutes, and discard the supernatant. Gently blow and beat with 1 ml of PBS, centrifuge at 1000 rpm for 5 minutes, and discard the supernatant. Repeat the operation once. Resuspend the cells with 200 μl of precooled 70% ethanol, and fix the cells in a 4°C refrigerator for at least 4 hours. Collect the cells by centrifuge, wash the cells twice with 1 ml of precooled PBS and centrifuge again, and discard the supernatant. Prepare the dye under dark conditions. Add 500 μl of propidium iodide staining solution to each tube of sample and mix well, incubate at 37°C for 30 minutes, and then detect with a flow cytometer.s
Results
The Figure 1 shows the hysteresis return lines of Fe3O4 and ZIF-8-GO-Fe3O4. From the figure, it can be seen that both Fe3O4 and ZIF-8-GO-Fe3O4 exhibit zero coercivity and remanent magnetization, and they both possess good super para magnetism. The saturation magnetization of Fe3O4 and ZIF-8-GO-Fe3O4 is 42.15 emu/g and 21.29 emu/g, respectively, and although the saturation magnetization of ZIF-8-GO-Fe3O4 is reduced by nearly half, ZIF-8-GO-Fe3O4 is still sufficient for magnetically responsive drug delivery.
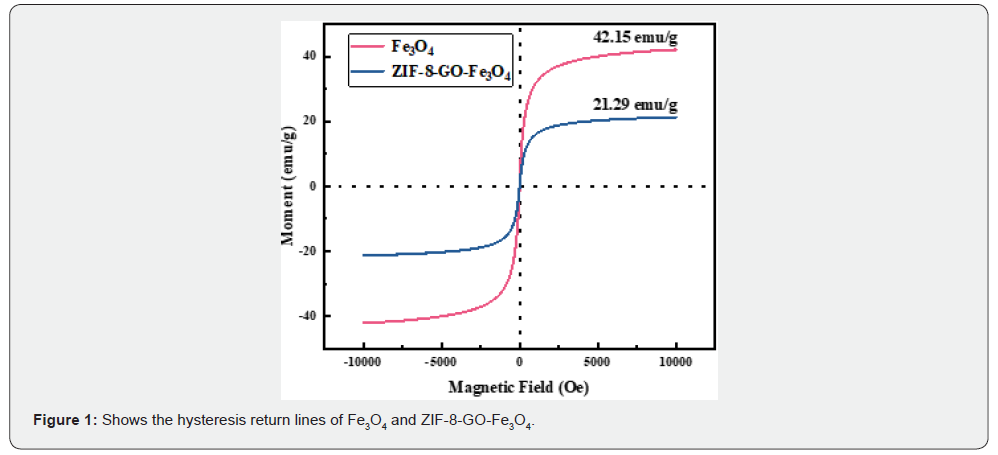
Figure 2(a, b) shows the scanning electron microscope image of GO-Fe3O4-ZIF-8. These SEM images of the surface morphology of the GO-Fe3O4-ZIF-8 showing the particles are mostly irregularly square and uniform in size. The images (a) were captured at a magnification of 50.0k×, and the scale bar corresponds to 500nm. The images (b) were captured at a magnification of 10.0k×, and the scale bar corresponds to 2μm.
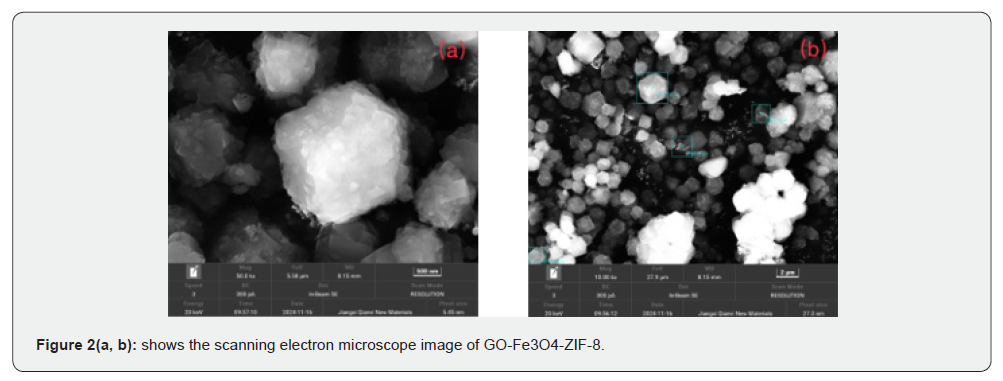
Cytotoxicity Experiment
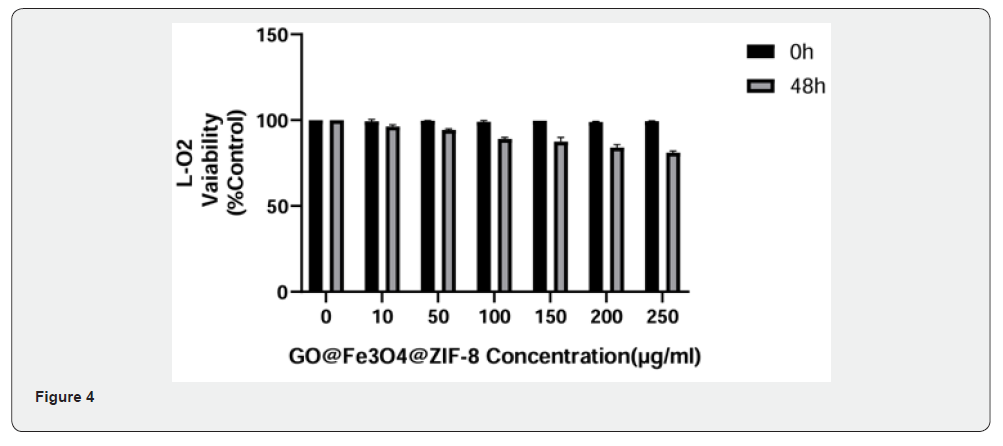
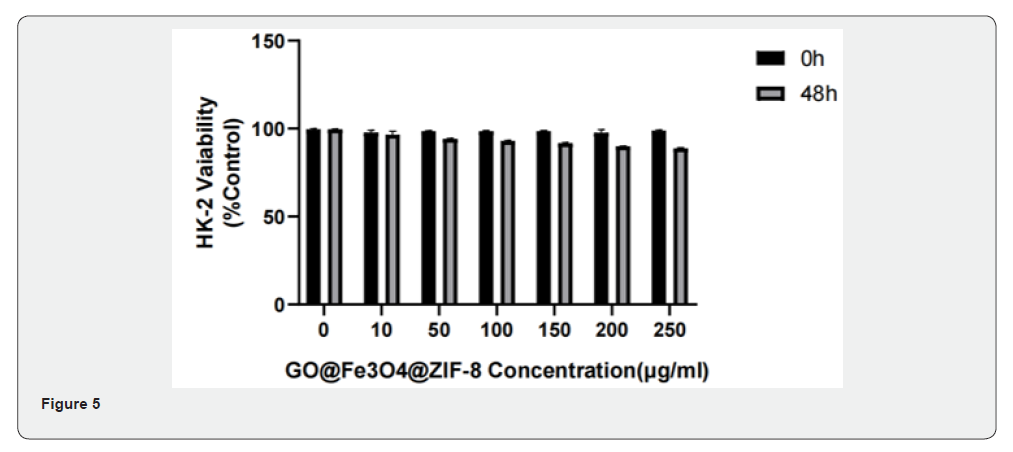
Figure 3-5 are the results of Cytotoxicity Experiment, which show the 48h survival rate of cells from different tissues after adding different concentrations of GO-Fe3O4-ZIF-8. The experimental results showed that when the carrier concentration was 10 μg/ml, the survival rates of normal breast cells, normal liver cells, and normal renal tubular cells were 99.45%, 94.92%, and 99.12%, respectively, and when the concentration was 250 μg/ml, the cell survival rates were 90.90%, 78.247%, and 89.18%, respectively.
Flow cytometry cell cycle experiment
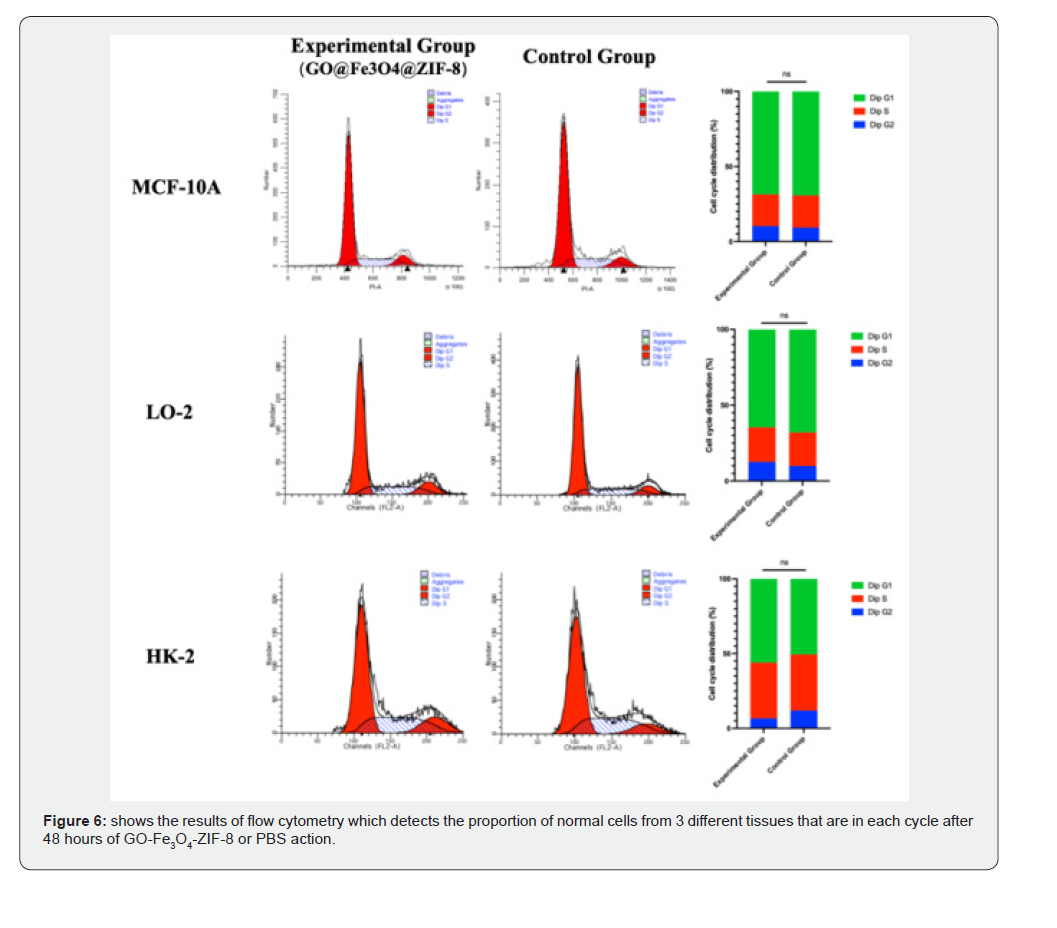
Figure 6 shows the results of flow cytometry, which detects the proportion of normal cells from 3 different tissues that are in each cycle after 48 hours of GO-Fe3O4-ZIF-8 or PBS action. The experimental results showed that there was no statistically significant difference in the proportions of the G0/G1, S, and G2/M phases between the GO-Fe3O4-ZIF-8 carrier group and the normal cell group.
Discussion
Cytotoxicity Experiment
Medical biomaterials need to have good biocompatibility. According to the result of Cytotoxicity Experiment, it can be seen that even if a higher concentration of GO-Fe3O4-ZIF-8 carrier was accumulated in normal cells of different tissues, the effect of this carrier on the cell survival rate was still small, and the GO-Fe3O4- ZIF-8 composites had good biocompatibility.
Flow cytometry cell cycle experiment
The existing research and discoveries show that drug-loaded particles have entered an era of rapid development, but there are very few drug carriers that are maturely used in clinical treatment [22]. The reason why various drug-loaded particles are all in the experimental stage is that drug carriers should avoid adverse reactions such as mutation, distortion and carcinogenesis of tissue cells as much as possible. Meanwhile, drug-loaded carriers need to follow the body fluid system instead of directly penetrating the barriers between cells, thus causing cell damage [23]. The drug carrier that is expected is to achieve local specific cancer treatment while minimizing the damage of drug-loaded particles to the body [24]. Therefore, we conducted a flow cytometry cell cycle experiment for verification. The experimental results suggest that the high concentration of the carrier does not affect the cell cycle process of normal tissue cells, nor does it cause mutation and distortion of tissue cells.
Conclusion
The GO-Fe3O4-ZIF-8 nanocarriers synthesized in this study were prepared in a simple manner with a stable structure. The good biocompatibility of GO-Fe3O4-ZIF-8 nanocarriers was confirmed by a series of cell experiments, which means that the GO-Fe3O4-ZIF-8 nanocarriers loaded with chemotherapeutic drugs have little effect on normal breast cells while killing breast cancer cells. The carrier will be transported with the blood to all parts of the body and metabolized in the liver and kidneys during practical application. In this study, it was confirmed that the survival rate of liver and kidney cells was still high even under the high concentration of GO-Fe3O4-ZIF-8 nanocarriers, which meets the needs of clinical application in breast cancer treatment and has a broad clinical application prospect.
Acknowledgments
This work was supported by applied basic research program of Science and Technology
Conflict of interest
The authors declare no competing financial interest.
References
- Siegel RL, Giaquinto AN, Jemal A (2024) Cancer statistics, 2024. CA Cancer J Clin 74(1): 12-49.
- Sarhangi N, Hajjari S, Heydari SF, Ganjizadeh M, Rouhollah F, et al. (2022) Breast cancer in the era of precision medicine. Mol Biol Rep 49(10): 10023-10037.
- Tenney S, Oboh-Weilke A, Wagner D, Chen MY (2024) Tamoxifen retinopathy: A comprehensive review. Surv Ophthalmol 69(1): 42-50.
- Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD (2011) cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 13(3): R64.
- Lei C, Han F, Li D, Li WC, Sun Q, et al. (2013) Dopamine as the coating agent and carbon precursor for the fabrication of N-doped carbon coated Fe3O4 composites as superior lithium-ion anodes. Nanoscale 5: 1168-1175.
- Qu Y, He F, Yu C, Liang X, Liang D, et al. (2018) Advances on graphene-based nanomaterials for biomedical applications. Mater Sci Eng C Mater Biol Appl 90: 764-780.
- Arabkhani P, Javadian H, Asfaram A, Ateia M (2021) Decorating graphene oxide with zeolitic imidazolate framework (ZIF-8) and pseudo-boehmite offers ultra-high adsorption capacity of diclofenac in hospital effluents. Chemosphere 271.
- Pandey RP, Kallem P, Hegab HM, Rasheed PA, Banat F, et al. (2022) Cross-linked laminar graphene oxide membranes for wastewater treatment and desalination: A review. J Environ Manage 317: 115367.
- Guerra ACS, de Andrade MB, Tonial Dos Santos TR, Bergamasco R (2021) Adsorption of sodium diclofenac in aqueous medium using graphene oxide nanosheets. Environ Technol 42(16): 2599-2609.
- Peng J, Tian H, Du Q, Hui X, He H (2018) A regenerable sorbent composed of a zeolite imidazolate framework (ZIF-8), FeO and graphene oxide for enrichment of atorvastatin and simvastatin prior to their determination by HPLC. Mikrochim Acta 185(2): 141.
- Sharma H, Mondal S (2020) Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int J Mol Sci 21(17): 6280.
- Zu Nurain Ahmad S, Salleh WNW, Yusof N, Yusop MZM, Hamdan R, et al. (2024) Efficiency of magnetic zeolitic imidazolate framework-8 (ZIF-8) modified graphene oxide for lead adsorption. Environ Sci Pollut Res Int .
- Wang Q, Sun Y, Li S, Zhang P, Yao Q (2020) Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv 10(62): 37600-37620.
- Zhou R, You Y, Zha Z, Chen J, Li Y, et al. (2023) Biotin decorated celastrol-loaded ZIF-8 nano-drug delivery system targeted epithelial ovarian cancer therapy. Biomed Pharmacother167: 115573.
- Ge Y, Wang K, Liu J, Tian Y, Li H, et al. (2022) A ZIF-8-based multifunctional intelligent drug release system for chronic osteomyelitis. Colloids Surf B Biointerfaces 212: 112354.
- Obaid EAMS, Wu S, Zhong Y, Yan M, Zhu L, et al. (2022) pH-Responsive hyaluronic acid-enveloped ZIF-8 nanoparticles for anti-atherosclerosis therapy. Biomater Sci 10: 4837-4847.
- Chen L, Peng J, Wang F, Liu D, Ma W, et al. (2021) ZnO nanorods/FeO-graphene oxide/metal-organic framework nanocomposite: recyclable and robust photocatalyst for degradation of pharmaceutical pollutants. Environ Sci Pollut Res Int 28(17): 21799-21811.
- Gostaviceanu A, Gavrilaş S, Copolovici L, Copolovici DM (2024) Graphene-Oxide Peptide-Containing Materials for Biomedical Applications. Int J Mol Sci 25(18): 10174.
- Zabé N, Foo K, Hashim U, Tan S, Liu W (2017) Procedia Engineering 184: 469-477.
- M M A B, ABDELBAKI B, DINA E, et al. (2022) JOM 74: 3531-3539.
- Kida K, Okita M, Fujita K, Tanaka S, Miyake Y (2013) CrystEngComm 15: 1794-1801.
- Ajith S, Almomani F, Elhissi A, Husseini GA (2023) Nanoparticle-based materials in anticancer drug delivery: Current and future prospects. Heliyon 9(11): e21227.
- Vasić K, Knez Ž, Leitgeb M (2024) Multifunctional Iron Oxide Nanoparticles as Promising Magnetic Biomaterials in Drug Delivery: A Review. J Funct Biomater15(8): 227.
- Kashyap BK, Singh VV, Solanki MK, Kumar A, Ruokolainen J, et al. (2023) Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega 8(16): 14290-14320.