Abstract
In this work, Zinc Oxide (ZnO) nanostructures deposited on Fluorine Doped Tin-Oxide (FTO) substrate and zinc foil substrate using electrochemical deposition techniques. The prepared samples were calcinated at 400°C (30 minutes) for controlling finalized the properties. Among various characterization techniques, selected field emission scanning electron microscopy to know the morphological investigation at nanoscale level. The FESEM nanograph shows the well- defined and uniformly grown nanostructures (nanorod, nanowire and cauliflower-like structures) observed. It has confirmed the diameter of the nanorods/FTO), cauliflower-like structure/Zn foil. These nanostructures diameter varies at 167 nm and 23 nm. In future, these nanostructures used as photoanodes in dye sensitized solar cell devices.
Keywords: ZnO; Electrodeposition; FESEM; Nanorod; Nanowire
Introduction
ZnO has wider bandgap and attracted for various industries applications, including smart devices, sensors, dye-sensitized solar cells, photocatalysis etc. This ZnO nanostructure has various unique properties like resistivity control, direct bandgap, transmittance in the visible spectral region along with that large surface to volume ratio etc [1]. Among various methods, electrodeposition method is suitable by tailoring structural, morphological and optical properties. Recently, Sigamani Saravanan [2] presented electrochemical deposition of ZnO and Zn-doepd TiO2 thin film on FTO substrate. They reported the hexagonal wurtzite structure and well matched JCPDS file and AFM topography revealed the surface roughness of 280 nm [2]. Extensively, ZnO nanostructure used as photoanode in dye sensitized solar cells which was prepared by electrochemical deposition method. The demonstration of ZnO nanostructure deposited on FTO at distinct potential and varied the energy bandgap. These DSSC cell achieved around 1.2% of conversion cell efficiency [3].
Materials and Methods
Materials
For this deposition process, Zn foil, FTO glass, Zinc Chloride (ZnCl2), De-Ionized (DI) water, Acetone (C3H6O) and Methanol (CH3OH) and Potassium Chloride (KCl) are procured. In that ZnCl2 acting as primary source of the materials.
Method
The electrochemical method selected with the chronopotentiometry mode with computer-controlled potentiostat as a potential source. These electrochemical deposition processes were carried out by using 1x10-3 mol/L of ZnCl2 and 0.1 mol/L of KCl. Initially, the above materials are dissolved invidually de-ionized water of 100 ml. These techniques employed the three different electrodes configuration set-up. Next, zinc foil used as working electrode to deposit the atoms or molecules, Platinum (Pt) wire as the counter electrode and Ag/AgCl as the reference electrode. During the deposition process, maintained -1.4 V as applied potential with the deposition processing time was 30 minutes. However, the Oxygen (O2) flow kept throughout the deposition process along continuous stirring with the constant temperature at 80 °C maintained.
Results and Discussion
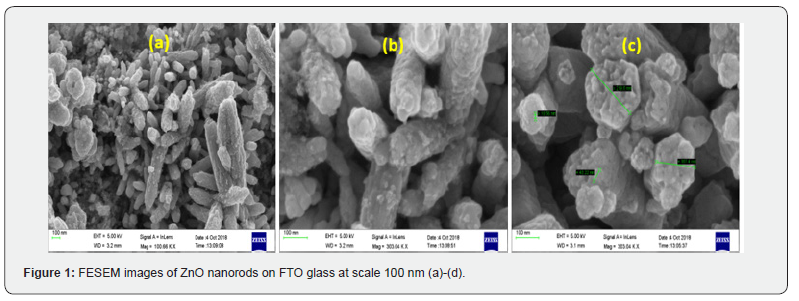
The zinc oxide thin film deposition on conductive surface using electric current and redox chemical reactions. The preparation method and substrates are significantly affect the morphology, size and properties ZnO nanostructures. Figure 1(ac) shows the morphological images of zinc oxide thin film on FTO substrate using FESEM techniques. With the deposition process, ZnO nanostructure growth various on FTO glass substrate like nanorods and hexagonal images at the magnification of 100, 303 KX. The free nanorods standing on the FTO substrate and interesting for various applications such as dye sensitized solar cells. These are 1D ZnO nanorods maintaining uniform diameter and length at nanoscale range. Also observed some agglomeration in Figure 1(a). The perfectly hexagonal shape nanorods observed with the flot-topped view of the as-grown ZnO nanorods. It has diameters around 168 nm as depicted in Figure 1(b-c). Furthermore, calculated the grain sizes which is varied from 20 to 44 nm. Overall, the morphological image shows freestanding nanorods of ZnO on FTO glass substrate due to greater control over their growth [4]. As compared to Figure 1(a-c), we can notice different morphological images of the ZnO nanostructures on zinc foil as depicted in Figure 2(a-c). It is clearly visible and welldeposited flower natured morphology. Here, these nanographs (images) are needle on cauliflower-like structures [5]. These ZnO cauliflower-like structure grain diameters were calculated as 22.95, 50.41, and 76.4 nm. The FESEM image observations of this cauliflower-like nanostructure. Fascinatingly, some of the small needle like structure are assembled at the top of the cauliflower structure. These structures are perpendicular to the substrate surfaces. The deposited nanostructures are uniformly on zinc foil substrates. These evaluations exploring the difference nucleation and growth mechanism between ZnO and Zn foil substrates.

Conclusion
In this summary, focused on electrochemical deposition of ZnO nanostructures grown on two different substrates and calcinated 400 °C for 1 hour. The morphological studies were observed from field emission scanning electron microscopy. It was revealing the nanorods on FTO substrates and cauliflowerlike structure on zinc foil substrates. These surface morphology images showed the well-defined and uniformly grown nanorods, and nanocauliflower-like structures. In near future, the optical, and structural properties could be utilized for dye-sensitized solar cell applications.
References
1. Altaf CT, Colak TO, Karagoz E, Wangm J, Liu Y, et al. (2024) Co-sensitization of Copper Indium Gallium Disulfide and Indium Sulfide on Zinc Oxide Nanostructures: Effect of Morphology in Electrochemical Carbon Dioxide Reduction. ACS Omega 9(17): 19209-19218.
2. Saravanan S, Dubey RS, Sarma GVS (2023) Electrochemically deposited ZnO and sol-gel spin-coated Zn-doped TiO2 thin films on FTO: an optical investigation. ES Energy & Environment 22: 1014.
- Dubey RS, Saravanan S (2020) Electrochemical growth and characterisation of ZnO nanostructures for dye‐sensitised solar cells. Micro & Nano Letters 15(12): 840-844.
- Clarke B, Ghandi K (2023) The interplay of growth mechanism and properties of ZnO nanostructures for different applications. Small 19(44): e2302864.
- Umar A, Akhtar MS, Almas T, Ibrahim AA, Assiri AMS, et al. (2019) Direct growth of flower-shaped ZnO Nanostructures on FTO substrate for Dye-sensitized solar cells. Crystals 9(8): 405.