Abstract
Nanomedicine is revolutionizing healthcare by enabling targeted therapies, advanced diagnostics, and personalized treatment strategies. By 2025, significant progress has been made in smart drug delivery systems, mRNA and gene delivery via lipid nanoparticles, and theranostic platforms that combine treatment and real-time imaging. Innovations in nanosensors and wearable devices are enhancing continuous health monitoring and early disease detection. The integration of artificial intelligence, biodegradable nanomaterials, and bioinspired systems is improving safety, precision, and sustainability. As nanomedicine moves closer to clinical reality, its future will be shaped by interdisciplinary innovation, digital health convergence, and equitable access to nano-enabled solutions. This review summarizes recent advances and outlines key future directions in this rapidly evolving field.
Keywords: Nanomedicine; Targeted drug delivery; mRNA Therapeutics; Theranostics; Nanosensors; Lipid nanoparticles; Neurodegenerative disorders; Covid-19; Toxicity; Scalability; Pharmacokinetics; Diagnosis; Biotechnology
Introduction
Nanomedicine, a convergence of nanotechnology and medical science, represents one of the most dynamic and innovative areas of modern healthcare. By manipulating materials at the nanoscale (1–100 nm), researchers have unlocked unique physical, chemical, and biological properties that allow for unprecedented precision in diagnosis, therapy, and disease monitoring [1]. Over the past two decades, nanomedicine has rapidly evolved from a conceptual framework to a multidisciplinary research field with tangible clinical applications. The field’s momentum has been fueled by advances in material science, biotechnology, and computational modeling, as well as by urgent global health needs such as cancer, infectious diseases, and neurodegenerative disorders [2]. Unlike traditional pharmaceuticals, nanomedicine platforms can be tailored to achieve controlled drug release, site-specific targeting, enhanced solubility of hydrophobic drugs, and improved pharmacokinetics [3]. Nanocarriers can bypass biological barriers, reduce systemic toxicity, and enhance therapeutic efficacy-benefits that have revolutionized drug delivery systems and imaging techniques. In recent years, the successful deployment of lipid nanoparticles (LNPs) for mRNA vaccine delivery, particularly during the COVID-19 pandemic, has highlighted nanomedicine’s clinical potential on a global scale [4]. This milestone not only validated the concept of nanoscale drug delivery but also catalyzed investment, research, and regulatory interest in nano-enabled therapies [4]. Moreover, innovations in nanosensors [5], theranostics [6], nanorobots [7] and bio responsive systems [8] are pushing the boundaries of what is possible in disease treatment and monitoring [9]. In 2025, the focus of nanomedicine is increasingly shifting from proof-of-concept studies to clinical translation and commercialization. Researchers and clinicians are working collaboratively to overcome key challenges, including biocompatibility, toxicity, scalability, and regulatory standardization [10]. This mini-review provides a concise overview of the recent advancements, clinical milestones, challenges, and future directions that define the rapidly evolving landscape of nanomedicine (Figure 1).
Recent Advances in Nanomedicine
Nanomedicine has matured significantly over the past few years, with several key innovations enhancing both the efficacy and safety of medical interventions. In 2025, the focus is on developing more intelligent, targeted, and responsive nanoplatforms. Below are the most notable recent advances:
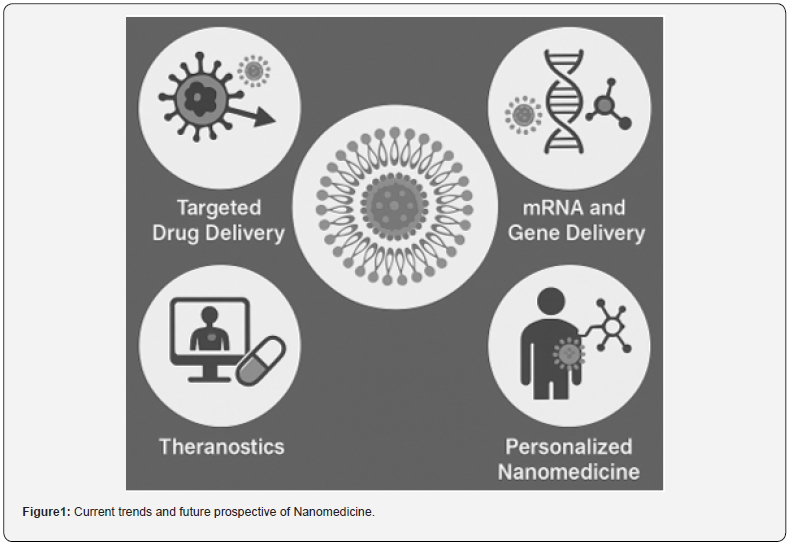
Targeted Drug Delivery
Targeted drug delivery remains at the heart of nanomedicine. By engineering nanoparticles to recognize and bind specific cellular markers, drugs can be delivered directly to diseased tissues, sparing healthy cells and minimizing side effects [11]. Advances in ligand engineering-such as antibody fragments, peptides, and aptamers-have improved the specificity of nanocarriers for tumors, inflamed tissues, and infection sites. In oncology, stimuli-responsive nanocarriers have gained attention. These nanoparticles can release their payload in response to specific internal stimuli (e.g., pH, redox gradients, or enzymatic activity) or external triggers (e.g., heat, light, or magnetic fields). For instance, acid-sensitive liposomes loaded with doxorubicin are being used to exploit the acidic microenvironment of solid tumors for site-specific release [12].
mRNA and Gene Delivery
The success of mRNA vaccines during the COVID-19 pandemic has propelled lipid nanoparticles (LNPs) to the forefront of nucleic acid delivery systems. In 2025, LNPs are being optimized for stability, tissue targeting, and immune evasion, expanding their application to cancer immunotherapy, rare genetic disorders, and infectious disease prevention [13]. Beyond mRNA, gene-editing tools like CRISPR-Cas9 are being integrated into nanoparticle platforms. Nanocarriers designed for nucleus-specific delivery of CRISPR components have demonstrated promising results in correcting genetic mutations in preclinical models. These platforms are now entering early-phase human trials for conditions like Duchenne muscular dystrophy and sickle cell disease [14].
Theranostics
Theranostic nanomedicine-combining therapy and diagnostics in a single platform-is an emerging paradigm. Gold nanoparticles, magnetic nanoparticles, and quantum dots are being used to simultaneously deliver drugs and track treatment responses via imaging modalities like MRI, CT, and fluorescence [15]. In particular, superparamagnetic iron oxide nanoparticles (SPIONs) have found applications in tumor imaging and hyperthermiabased cancer therapy. Multifunctional nanoparticles carrying both imaging agents and chemotherapeutics enable real-time monitoring of drug delivery and biodistribution, improving personalized treatment strategies [16].
Smart Nanosensors and Wearables
Nanosensors are redefining diagnostics by enabling ultrasensitive, point-of-care detection of biomarkers related to cancer, cardiovascular disease, and neurodegeneration [17]. Integrated into wearable devices, these nanosensors can continuously monitor physiological states such as glucose levels, inflammatory markers, and even circulating tumor DNA. Emerging implantable nanosensors systems provide real-time monitoring of treatment efficacy and disease recurrence, paving the way for responsive and autonomous therapeutic systems [18].
Regulatory Progress and Clinical Translation
A notable recent trend is the increase in regulatory approvals for nanomedicine-based therapies and diagnostics. Improved frameworks by the FDA, EMA, and other agencies are streamlining evaluation procedures, encouraging clinical trials and accelerating market access. Clinical-grade nanomedicines are now used for cancer, ocular diseases, and vaccine delivery, demonstrating both safety and efficacy [19].
Clinical and commercial landscape
The translational success of nanomedicine is no longer limited to the laboratory. As of 2025, numerous nanotechnologybased products have entered clinical use, while others are in latestage clinical trials, demonstrating the field’s growing maturity and commercial viability. Pharmaceutical companies, biotech startups, and research institutions are actively investing in nanomedicine pipelines, driven by both therapeutic demand and market potential [20].
Approved Nanomedicine Products
Several nanomedicine formulations have received regulatory
approval from the U.S. Food and Drug Administration (FDA),
European Medicines Agency (EMA), and other agencies. These
include:
• Doxil® (liposomal doxorubicin): The first FDA-approved
nanodrug, used for treating ovarian cancer and Kaposi’s sarcoma.
Its PEGylated liposome formulation enhances circulation time
and reduces cardiotoxicity [21].
• Abraxane® (albumin-bound paclitaxel): An example of a
protein-based nanomedicine that improves solubility and tumor
uptake in metastatic breast cancer [22].
• Onpattro® (patisiran): A lipid nanoparticle-based siRNA
drug used to treat hereditary transthyretin-mediated amyloidosis.
It represents a milestone in RNA interference therapy [23].
• Comirnaty® (Pfizer–BioNTech COVID-19 vaccine): The
global success of this mRNA-LNP vaccine has validated the use of
nanotechnology for rapid and scalable vaccine development [24].
These approved products serve as proof of concept for nanomedicine’s therapeutic value and have encouraged further research into nanoplatforms for less common and more complex diseases.
Clinical Trials and Pipeline Developments
As of 2025, hundreds of nanomedicine formulations are under
evaluation in clinical trials [25]. Key areas of focus include:
• Cancer immunotherapy: Nanoparticles are being
designed to deliver tumor antigens and immune adjuvants to
dendritic cells, stimulating a potent anti-tumor immune response
[26].
• Neurodegenerative diseases: Engineered nanoparticles
capable of crossing the blood-brain barrier (BBB) are under
development for treating Alzheimer’s and Parkinson’s diseases
[27].
• Infectious diseases: Beyond COVID-19, nano vaccines
and antiviral nanocarriers are being explored for HIV, tuberculosis,
and malaria [28].
Startups and large companies alike are investing in scalable manufacturing, bioinformatics-driven design, and regulatory documentation to accelerate commercialization. Moreover, publicprivate partnerships are emerging to support nanomedicine infrastructure, particularly in low- and middle-income countries.
Challenges and limitations
Despite its remarkable progress, nanomedicine faces a range of scientific, technical, regulatory, and economic challenges that hinder its full clinical integration. These limitations need to be systematically addressed to realize the field’s long-term potential and ensure safe, equitable access to nano-enabled therapies.
Biocompatibility and Toxicity
A major concern in nanomedicine is the potential toxicity of nanomaterials. Nanoparticles may accumulate in nontarget tissues such as the liver, spleen, and kidneys, leading to unintended side effects. Long-term exposure risks, such as chronic inflammation, immunogenicity, or oxidative stress, are not yet fully understood for many nanomaterials, especially inorganic ones like gold, carbon-based nanostructures, or metal oxides. In vitro models often fail to fully predict in vivo behavior, and traditional toxicity assays are not always applicable at the nanoscale. There is a pressing need for standardized protocols to assess biodistribution, metabolism, excretion, and long-term safety of nanotherapeutics [29].
Regulatory Uncertainty
Regulatory pathways for nanomedicines remain complex and underdeveloped. Many nano formulations do not fit neatly into existing drug, biologic, or device categories. Agencies like the FDA and EMA have issued guidance, but significant ambiguity persists regarding classification, clinical trial design, and product labeling. Multifunctional nanoplatforms that combine diagnostic and therapeutic components (theranostics) are particularly challenging to regulate due to their dual nature. Harmonizing global regulatory frameworks and establishing nanoparticlespecific quality and safety standards is crucial for expediting clinical approval [30].
Scalability and Manufacturing
The production of nanomedicine products at an industrial scale while maintaining batch-to-batch consistency poses significant technical hurdles. Variability in nanoparticle size, surface charge, drug loading, and stability can affect clinical efficacy and safety. Moreover, the manufacturing processes often involve complex, sensitive steps (e.g., emulsification, extrusion, solvent removal) that require precise control and high-cost infrastructure. Efforts are underway to adopt continuous-flow synthesis, automation, and quality-by-design (QbD) approaches to overcome these limitations, but widespread implementation is still limited [31].
Cost and Accessibility
Nanomedicines, especially those involving complex production processes or rare materials, tend to be more expensive than conventional therapies. This restricts their availability in lowresource settings and raises concerns about healthcare equity. Balancing innovation with affordability is essential to prevent a technological divide in global healthcare system [32].
Future Directions
As nanomedicine moves from conceptual promise to clinical reality, the next frontier lies in designing smarter, safer, and more accessible nanotechnologies. The future of this field will be shaped by interdisciplinary innovation, regulatory evolution, and a stronger emphasis on personalized and preventive medicine.
Personalized Nanomedicine
One of the most promising developments is the integration of nanomedicine with precision medicine. By tailoring nanoparticles to the genetic, proteomic, and metabolic profiles of individual patients, treatment efficacy can be significantly improved while minimizing adverse effects. Personalized nanomedicine platforms will leverage high-throughput screening, artificial intelligence (AI), and machine learning to predict optimal nanoparticle designs for specific patient subgroups.
Bioinspired and Biodegradable Nanomaterials
Future nanocarriers are likely to mimic natural biological systems, such as exosomes, viruses, or cellular membranes, to improve biocompatibility and targeting. These bioinspired nanocarriers may evade immune surveillance, enhance uptake by specific tissues, and degrade harmlessly after completing their therapeutic mission. The development of fully biodegradable nanomaterials, such as poly (lactic-co-glycolic acid) (PLGA), chitosan, and lipid-based systems, will also be essential to reduce long-term toxicity and environmental concerns associated with persistent nanostructures.
Convergence with Digital Health and AI
Nanomedicine is increasingly intersecting with digital health, especially through nanosensors and wearable devices that monitor biomarkers in real-time. These platforms can provide early diagnosis, track disease progression, and enable responsive drug delivery. Integration with mobile health applications and cloud-based analytics will facilitate remote patient management. Moreover, AI-driven nanoparticle design is becoming a transformative tool. Machine learning models can predict nanoparticle behavior, optimize formulations, and accelerate preclinical screening, dramatically reducing the cost and time of development.
Global Equity and Sustainable Nanomedicine
To ensure global impact, the field must prioritize scalable, cost-effective, and environmentally sustainable nanomedicine solutions. Efforts to democratize access-such as open-source nanomedicine platforms, decentralized manufacturing, and global collaborative frameworks-will be vital for delivering nanoenabled healthcare to underserved populations (Table 1).
Conclusion
Nanomedicine, in 2025 stands at a pivotal crossroads between breakthrough innovation and broad clinical adoption. With advances in targeted drug delivery, mRNA therapeutics, and theranostics, the field has demonstrated transformative potential in addressing some of the most pressing medical challenges of our time. The recent clinical successes-such as lipid nanoparticlebased vaccines and siRNA therapies-have validated the promise of nanoscale interventions and accelerated investment, research, and policy attention. However, several challenges must be addressed to fully realize the impact of nanomedicine. Concerns regarding long-term safety, regulatory clarity, large-scale manufacturing, and equitable access continue to shape the research and commercialization landscape. Interdisciplinary collaboration among chemists, engineers, biologists, clinicians, and regulatory bodies will be key to overcoming these hurdles. Looking forward, the integration of nanotechnology with personalized medicine, artificial intelligence, and digital health tools will redefine how diseases are diagnosed, treated, and monitored. Sustainable and bioinspired nanomaterials, coupled with smart design principles, will usher in a new era of safe, accessible, and patient-centric healthcare. In conclusion, nanomedicine is no longer a futuristic vision but a rapidly evolving reality that holds immense promise for reshaping modern medicine. With strategic investments, global collaboration, and ethical foresight, nanomedicine can lead the way toward a healthier and more equitable future.
References
- Morozova O (2025) Advancements and Perspectives in Nanotechnology and Nanomedicine. Viruses 17(5): 638.
- Meng Y, Sui L, Xu T, Zhao H, Yuan Q, et al. (2025) Research and Application Prospect of Nanomedicine in Kidney Disease: A Bibliometric Analysis From 2003 to 2024. International Journal of Nanomedicine 20: 3007-3030.
- Caizer C (2024) Special Issue on Nanoparticles in Nanobiotechnology and Nanomedicine. International Journal of Molecular Sciences 26(1): 267.
- Mamidi N, De Silva FF, Salehi OM (2025) Advanced disease therapeutics using engineered living drug delivery systems. Nanoscale 17: 7673-7696.
- Aziz A, Noor U, Rana U, Qazalbash AA, Noor M, et al. (2025) Advanced nano sensors for smart healthcare. In Advanced Sensors for Smart Healthcare 329-345.
- DT Freire, JA Miranda, D Dourado, ÉDN Alencar (2025) Evolution of Theranostic Nanoparticles Through the Lens of Patents. Journal of Nanotheranostics 6(2): 11.
- I N Weerarathna, P Kumar, H Y Dzoagbe, L Kiwanuka (2025) Advancements in Micro/Nanorobots in Medicine: Design, Actuation, and Transformative Application. ACS omega 10(6): 5214-5250.
- E E Rani, D S Sanjana, E Karthikeyan, J Nandhini (2025) Smart Nanomaterials: Current State and Future Prospects in Drug Delivery and Tissue Engineering. Biomedical Materials & Devices 1-28.
- S Nikazar, H Haji‐Hashemi (2025) Nanomedicine-History and Recent Trends. Nanoengineered Materials for Medical and Healthcare Applications 1-24.
- T Chandy, C P Sharma (2025) An introduction to nanomedicine-past, present, and future. In Nanomedicine in Translational Research 3-16.
- AM Mahmoud, C Deambrogi (2025) Advancements in nanotechnology for targeted and controlled drug delivery in hematologic malignancies: shaping the future of targeted therapeutics. Applied Biosciences 4(1): 16.
- M Hassanzadeh-Khanmiri, A Moshari, R Kheradmand, T Haghgouei, M Homaei, et al. (2025) Nanomedicine: a cost-effective and powerful platform for managing neurodegenerative diseases. Metabolic Brain Disease 40(3): 142.
- Dwivedi J, Wal P, Ganesan S, Sharma A, Sharma P, et al. (2025) Messenger RNA Nanomedicine: Innovations and Future Directions. Curr Protein Pept Sci.
- Hadole P, Nikam HM, Avinash Gite, Pratik Kamble, Umesh jadhav (2025) Nanomedicine based approaches on mRNA delivery. International Journal of Scientific Research and Technology 2: 1.
- Shinde S, Shah S, Famta P, Wagh S, Pandey G, et al. (2025) Next-Generation Transformable Nanomedicines: Revolutionizing Cancer Drug Delivery and Theranostics. Molecular Pharmaceutics.
- X Yang, J Hu, Q Gao, Y Deng, Y Liu, et al. (2025) Advances in nano-delivery systems based on diagnosis and theranostics strategy for atherosclerosis. Journal of Drug Targeting 33(4): 492-507.
- J Sayyad, K Attarde (2025) Synergizing Nanotechnology and Artificial Intelligence for Society 5.0 Advancement Through Intelligent Systems. In Nano Mind: Exploring Synergies in Nanotechnology and Machine Learning pp 225-249.
- B Singh, A Nayyar (2025) Transforming healthcare through advanced sensing technologies. In Sensor Networks for Smart Hospitals pp17-40.
- P M Arvejeh (2024) Nanobiomaterials & nanomedicine. Journal of Translational Medicine 22(1): 1154.
- UT Khatoon, A Velidandi (2025) An Overview on the Role of Government Initiatives in Nanotechnology Innovation for Sustainable Economic Development and Research Progress. Sustainability 17(3): 1250.
- AA Gabizon, S Gabizon-Peretz, S Modaresahmadi, N M La-Beck (2025) Thirty years from FDA approval of pegylated liposomal doxorubicin (Doxil/Caelyx): an updated analysis and future perspective. BMJ oncology 4(1): e000573.
- S Shrestha, A Shrestha, J Kim, R K Thapa, JO Kim (2025) Recent advances in albumin nanoparticle-based cancer therapies. Journal of Pharmaceutical Investigation 55(1): 1-14.
- O Ebenezer, AK Oyebamiji, JO Olanlokun, JA Tuszynski, GKS Wong (2025) Recent Update on siRNA Therapeutics. International Journal of Molecular Sciences 26(8): 3456.
- RR Tjandrawinata, HS Budi (2025) Future Trends in Biotechnology Patents: Impact of Nano-Biomedicine and Artificial Intelligence on the Patent Landscape. Journal of Law, Politic and Humanities 5(3): 1571-1583.
- R Vecchio, L Gentile, S Tafuri, C Costantino, A Odone (2024) Exploring future perspectives and pipeline progression in vaccine research and development. Ann Ig 36(4): 446-461.
- H Bendani, N Boumajdi, L Belyamani, A Ibrahimi (2025) Revolutionizing breast cancer immunotherapy by integrating AI and nanotechnology approaches: review of current applications and future directions. Bioelectronic Medicine 11: 13.
- VK Yadav, S Dhanasekaran, N Choudhary, D Nathiya, V Thakur, et al. (2025) Recent advances in nanotechnology for Parkinson’s disease: diagnosis, treatment, and future perspectives. Frontiers in Medicine 12: 1535682.
- MR Babu, SA Rahaman, G Venkateswarlu, Y Mishra, V Mishra (2025) Nanomedicine research, development, and current clinical status. In Intelligent Nano biosystems in Medicine and Healthcare 1: 21-48.
- SA Arabiyat (2025) Prospectus and Concerns of Immunomodulatory Nanotechnologies and Nanoparticles Biocompatibility and Toxicity. In Nanotechnology Based Microbicides and Immune Stimulators pp165-189.
- U Manzoor, S Masood, S Nazir, L Younis, A Waheed, et al. (2025) Limitations and Concerns of Nanotechnology in Obtaining the Desirable Products. In Nanotechnology Based Microbicides and Immune Stimulators pp. 217-236.
- M Eyube, C Enuesueke, M Alimikhena (2025) The Future of Nanomaterials in Manufacturing.
- de Souza Cardoso Delfino C, de Paula Pereira MC, dos Santos Oliveira M, de Carvalho Favareto I, Valladão VS, et al. (2025) Scaling nano pharmaceutical production for personalized medicine: challenges and strategies. Journal of Nanoparticle Research, 27(4): 1-19.