Abstract
We present a fast and highly efficient approach for the preparation of pure phase α-Bi2O3 nanoparticles using microwave irradiation method at a temperature of 1600C. The calcination was done at temperatures of 2500C to 4500C. The synthesized particles were having size of around 40±20nms.The overall process is based on a reaction of bismuth nitrate pentahydrate, ethylene glycol and de-ionized water at very low temperature under magnetic stirring in stainless steel autoclave with a Teflon liner. The obtained samples were characterized with respect to morphology, crystal structure and composition by Field Emission Scanning Electron Microscopy (FESEM) and X-ray diffraction (XRD). The optical properties were discussed by means of UV, FTIR and Raman spectroscopy. The synthesized particles can be used for various applications in nanomedicine.
Keywords: Microwave synthesis; α-Bi2O3 nanoparticles; Structural studies; Optical properties
Abbreviations: FESEM: Field Emission Scanning Electron Microscopy; XRD: X-ray diffraction
Introduction
Bismuth oxide (Bi2O3) is a versatile and important metal oxide due to properties like wide energy gap change (from 2 to 3.96 eV), high oxide-ion conducting properties, large refractive index, dielectric permittivity (εr=190), high level of photoconductivity and photoluminescence [1]. Bismuth oxide nanoparticles have many applications in nanomedicine. They can treat multidrug-resistant bacteria by interacting with the bacterial cell wall, generating reactive oxygen compounds, and limiting biofilm production. Bismuth oxide nanoparticles can be used as contrast agents for CT tomography and photoacoustic imaging and can be used as radiosensitizers to enhance the efficacy of radiation therapy for cancer treatment. Bismuth oxide nanoparticles can be functionalized and loaded with drugs to deliver those to specific cells or tissues and can be used as radiation shielding materials in medical imaging and radiation therapy. Bismuth oxide nanoparticles have potential for bone regeneration and can suppress tumor growth under NIR laser radiation. Many efforts were employed by many researchers to develop methods to control size and distribution in the preparation of nanostructures of Bi2O3. Further, upon the usage of microwave irradiation [2], the rate of synthesizing nanomaterials is very fast and efficient in saving energy consumption as well as time. The band gap of the material plays vital role in various photocatalytic applications [3] like Photodegradation of toxic dyes. At current, many of synthetic routes including hydrothermal, sol-gel, precipitation, flame spray pyrolysis, solvo-thermal, and physical methods have been extensively used for synthesizing Bi2O3 nanoparticles.
Normally Bi2O3 is prepared by oxidizing bismuth metal at 8000C or thermal decomposition of carbonates or hydroxides produced by adding hydrates to bismuth salt. The powders on calcinations yield the fine particles of Bi2O3 [4-6]. M Anilkumar et al. [4] reported a simple citrate gel process for the preparation of nanocrystalline Bi2O3 involving complexation of metal ions by poly functional carboxyl group, such as citric acid or tartaric acid having one hydroxyl group [4]. MM Patil et al. [7] reported a simple process of digestion of amorphous bismuth hydride gelunder refluxing condition at 1000C for the preparation of nano crystalline Bi2O3 [7]. The direct synthesis of bismuth oxide by the hydrolysis and condensation of bismuth nitrite in microwave field have been reported by Eva Bartonickova et al. [8].
Experiment
All the chemicals used in the experiment were of analytical Grade and utilized as without further purification. In the procedure, 2.245g of Bi (NO3)3·5H2O was dissolved in a 100mL beaker containing 10mL ethylene glycol under magnetic stirring until it has dissolved and mean while bismuth nitrate pentahydrate was not allowed to hydrolyze. And then 20mL of absolute ethyl alcohol was added in the above solution under constant stirring for 2 h to get solution. The above mixed solution was transferred into a stainless-steel autoclave with a Teflon liner (Figure1) of 80 mL capability, and heated at 160 °C for 8 h. Then the autoclave was cooled naturally, the filtration of precipitates was done and washed many times with deionized water and absolute ethyl alcohol and finally it was dried in the air at 60 °C for 10 h. The precursor was transferred into ceramic crucibles with covers, and put into a muffle furnace heated at temperature of 450 °C for 4 h to decompose precursor into Bi2O3, respectively. Phase purity & structural studies of the as-prepared samples were carried out by using powder X-ray diffractometer (Model D-8 Advance & Bruker AXS- XRD) with Cu-Kα radiations of wavelength 1.54056 Å and the morphological studies have been carried out by field emission scanning electron microscope FESEM (Hitachi S4800). The band gap and optical properties were recorded by using Perkin Elmer model Lamda 950 UV-Vis Spectrometer and Raman spectroscopy (Bruker RFS 27, Stand Alone FT Raman Spectrometer).
Instrumentation
The instrument used for synthesis was a stainless-steel autoclave with a Teflon liner as shown in (Figure1).

Results and discussion
X-ray Diffraction (XRD) Studies
(Figure 2) shows XRD patterns of α - Bi2O3 samples for 2ө ranging from 200 to 800. From the XRD pattern, it can be observed that the location of the diffraction peaks is in good agreement with those of monoclinic α - Bi2O3.The diffraction pattern reveals presence of diffraction peaks in XRD spectra of the samples at 2θ=22.06°, 26.09°, 27.57°, 28.76°, 30.37°, 33.03°, 35.62°, 37.83°, 55.98° corresponding to orientation of the (102), (002), (121), (012), (122), (212), (113), (041), (104), (322) and (241) planes to well-defined peaks. All the diffraction peaks could be clearly indexed to monoclinic phase of Bi2O3 agreed with the JCPDS No. 712274. No other crystalline phases of Bi2O3 or impurities were observed indicating a very high purity phase of α - Bi2O3. The relative intensity of the diffraction peak arising from (1 2 1) plane is stronger in phase pure α-Bi2O3 form and it reveals that more crystallites may be oriented along (1 2 1) direction. The sharpness of the diffracted peaks indicates the good crystallinity of the material. The as synthesized α- Bi2O3 nanoparticles have a diameter of 20-60 nm, which is in good agreement with the results calculated from Debye–Scherer formula. The mean crystallite sizes of α- Bi2O3 can be calculated from the XRD data using Debye– Scherer formula:
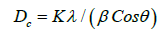
where β is the width of the observed diffraction peak at its half maximum intensity (FWHM), K is the shape factor, which takes a value of about 0.9 and λ is the X-ray wavelength (Cu Kα radiation equals to 1.54056 Å). displayed the mean crystallite size of the synthesized Bismuth nanoparticles which was estimated by the FWHM of the XRD peak (121) using the Debye Scherer equation.

Morphological studies:
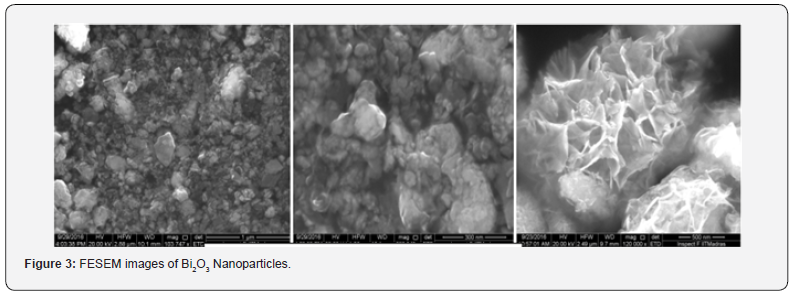
The morphological studies of the as synthesized nanoparticles were carried out by FESEM (Hitachi S4800). (Figure 3) shows the FESEM images of the different Bismuth oxide nanoparticles. It revealed more or less uniformity in the distribution of particles and well colligation [9]. The particles have well developed grain boundary. The images correspond to sample with a particle size in the range of 20 to 60 nm. The average particle size is around 50nm.
UV Visible Spectroscopy:
The optical absorption spectra of the samples were recorded by using Perkin Elmer model Lamda 950 UV-Vis Spectrometer in the range of 300-800nm and optical absorption coefficient has been calculated. (Figure 4(a)) shows the UV–Vis absorption spectra of the prepared samples. We studied the optical absorption of the as-prepared Bi2O3 nanoparticles to estimate its energy band gaps. The UV spectra of the synthesized Bi2O3 nanoparticles show the photo absorption properties from Ultraviolet light region to visible light with wavelength less than 470 nm. This is assigned to the intrinsic band gap absorption. The high slope shape of the spectra means that the light absorption was because of transition in band gap. This permits the as-prepared Bi2O3catalyst to respond to a wide range of solar spectrum and utilize visible light for photocatalysis. The absorbance decreases sharply indicating the presence of optical absorption in the region of 400- 500 nm. The indirect band gap is determined by extrapolating the linear portion of the plot and the band gap value is 2.75 eV which is equal to 450.9 nm. The band gap value of α- Bi2O3 is in good agreement with the value reported by Z Ai, et al. [4]. They synthesized monoclinic α- Bi2O3 via calcination of hydrothermally prepared bismuth oxide carbonate (BiO)2CO3 precursor at 5000C for 4 h and the band gap value was 2.72 eV. The band gap energies of the synthesized nanoparticles were calculated by Tauc Mott (TM) relation who relates absorption coefficient and the incident photon energy of semiconductors as:

Where α is the absorption coefficient, ‘a’ is a constant, and n is equal to 2 for allowed direct transitions and 0.5 for indirect transitions. (Figure 4(b)) shows the Tauc plots of samples and the band gap energy was estimated by extrapolation of the linear region. The band gap of the sample is 2.75 which is greater than the bulk Optical absorption coefficient (1.8-2.1 eV) [10-12].


Raman Spectroscopy:
Raman spectroscopic studies are helpful in differentiating the various phases of Bismuth and the Bismuth phase spectrum is different from those of common impurity phases, like magnetite and maghamite. (Figure 5) shows the Raman spectrum of the synthesized sample with major peaks observed at 93, 117, 136, 150, 159, 181, 209, 274, 314and 445 cm-1 and matches well with those in literature [13-16] Raman spectral studies conformed that obtained powder is single phase α-Bi2O3.Raman spectra show broad bands in the higher-frequency region which matches well for α- Bi2O3 [13]. 117 mode comes from Ag symmetry of Bi atoms.136 Ag mode and 150 Bg mode due to displacements of both Bi and O atoms in the α-Bi2O3 lattice.209, 274, 314, 445 and are attributed to the displacements of the O atoms in α-Bi2O3.

Conclusion
Nanoparticles of Bismuth oxide Bi2O3 were synthesized by hydrothermal method using microwaves in autoclave with bismuth nitrate pentahydrate, ethylene glycol and de-ionized was reactants and calcinated at temperature of 4500C. The synthesized particles were having size of around 50nms. XRD pattern reveals that the location of the diffraction peaks is in good agreement with those of monoclinic α - Bi2O3. The optical absorption of the as-prepared Bi2O3 nanoparticles is studied to estimate its energy band gap. The band gap is determined by Tauc plot and value is 2.75 eV which is equal to 451 nm (approx.). Raman spectra confirmed that obtained bismuth oxide Bi2O3 nanoparticles has single monoclinic α- phase and can be further used for applications in nanomedicine.
Acknowledgements
The authors would acknowledge Department of Science and Technology, New Delhi for financial support under Nano Mission Project at NIT Srinagar. Further the support of Prof C.N.R Rao at IIT Madras and Prof. Shubra Singh at Anna University Chennai is highly acknowledged for providing characterization instruments. Also, the support of Govt BHSS Kandi Karnah Kupwara is appreciated.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
References
- M Prekajski, A Kremenovic, B Babic, M Rosic, B Matovic, et al. (2010) Materials Letters 64(20): 2247-2250.
- Leontie L, Caraman M, Alexe M, Harnagea C (2002) Structural and optical characteristics of bismuth oxide thin films. Surface Science 507-510: 480-485.
- Wang Y, Zhao J, Zhu Y, Zhou B, Zhao X, et al. (2013) Colloids and Surfaces A: Physicochemical and Engineering Aspects 434: 296-302.
- Anilkumar M, Pasricha R, Ravi V (2005) Synthesis of bismuth oxide nanoparticles by citrate gel method. Ceramics international 31(6): 889-891.
- Krueger J, Winkler P, Luderitz E, Lck M, Wolf HU (1985) Bismuth, bismuth alloys, and bismuth compounds. Ullmann's Encyclopedia of Industrial Chemistry.
- Liu AT, Kleinschmidt P, Davidge RW (1986) Novel ceramic fabrication process and applications. Institute of Ceramics, Sta s, UK, 38: 1-10.
- Patil MM, Deshpande VV, Dhage SR, Ravi V (2005) Synthesis of bismuth oxide nanoparticles at 1000C. Materialsletters, 59(19): 2523-2525.
- Bartonickova E, Cihlar J, Castkova K (2007) Microwave assisted synthesis of bismuth oxide. Processing and Application of Ceramics 1(1-2): 29-33.
- L Zhou, WZ Wang, HL Xu, SM Sun, M Shang (2009) Bi2O3 Hierarchical Nanostructures: Controllable Synthesis, Growth Mechanism, and their Application in Photocatalysis. Chem Eur J 15(7): 1776-1782.
- L Zhou, WZ Wang, HL Xu, SM Sun, M Shang (2009) Bi2O3 Hierarchical Nanostructures: Controllable Synthesis, Growth Mechanism, and their Application in Photocatalysis. Chem Eur J 15(7): 1776-1782.
- S Sen, R N P Choudhary, P Pramanik (2007) Structural and electrical properties of Ca2+-modified PZT electroceramics. Physica B 387(1-2): 56-62.
- S Dutta, RN P Choudhary, P K Sinha, A K Thakur (2006) Microstructural studies of ceramics using complex impedance spectroscopy. Journal of Applied Physics 96: 1607-1613.
- Narang SN, Patel ND, Kartha VB (1994) Infrared and Raman spectral studies and normal modes of α-Bi2O3. J Mol Struct 327(2-3): 221-235.
- Betsch RJ, White WB (1978) Vibrational spectra of bismuth oxide and the sillenite-structure bismuth oxide derivatives. Spectrochim Acta Part A 34(5): 505-514.
- Popovic ZV, Thomsen C, Cardona M, Liu R, Stanisic G, et al. (1988) Phonon characterization of Bi2 (Sr1-xCax)2 CuO6+δ by infrared and Raman spectroscopy. Solid State Commun 66(9): 965-969.
- Crossley A, Graves PR, Myhra S (1991) Phys C 176: 10.