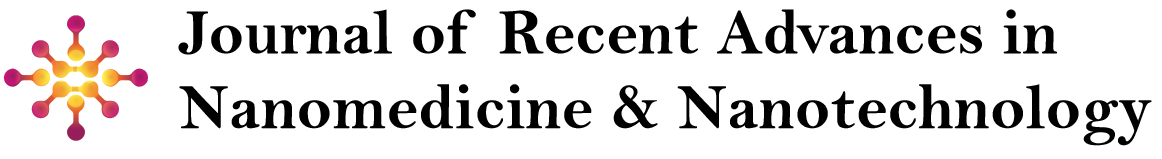Abstract
The development of nanoparticle systems for cancer drug delivery represents a promising strategy to overcome the limitations of conventional cancer therapies. Nanoparticles offer targeted delivery, reduced systemic toxicity, and improved bioavailability of chemotherapeutic agents. However, their translation from bench to bedside remains challenging due to several biological, technological, and regulatory barriers. This review discusses the recent advancements, challenges, and future opportunities in the field of nanoparticle-based cancer drug delivery systems. It highlights novel approaches, such as the use of biocompatible materials, multifunctional nanoparticles, and smart delivery systems, which enhance the efficacy and safety of cancer treatments.
Keywords: Cancer drug delivery; Dendrimers; Micelles; Protein-Based nanoparticles; Carbon-Based nanoparticles
Abbreviations: NDDSs: Nanoparticle-Based Drug Delivery Systems; SLNs: Solid Lipid Nanoparticles; PLGA: Poly (lactic-co-glycolic acid); PEG: Polyethylene Glycol; EPR: Enhanced Permeability and Retention; MPS: Mononuclear Phagocyte System; ROS: Reactive Oxygen Species
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, presenting significant challenges in its effective treatment [1]. Traditional therapeutic modalities, including chemotherapy and radiation, often have systemic effects that can damage healthy tissues, leading to adverse side effects and limiting the maximum tolerated dose. Moreover, drug resistance- phenomenon where cancer cells become less responsive to existing treatments-exacerbates the difficulty in managing various malignancies and further complicates treatment protocols [2]. The problems associated with conventional drug delivery systems primarily stem from their inability to selectively target tumor cells while sparing normal tissues. This non-specific distribution can result in substantial toxicities and reduced therapeutic efficacy. Additionally, many conventional therapies suffer from poor bioavailability and rapid elimination from the body, which necessitates frequent dosing and can affect treatment adherence [3-5]. The use of nanoparticle systems for drug delivery offers a novel approach to enhance the efficacy and selectivity of cancer therapies through targeted delivery and controlled release. Their unique properties, such as the ability to improve solubility, enhance the permeability and retention effect, and achieve precise targeting to tumor sites, significantly increase the therapeutic index of anticancer agents [6-8]. By engineering nanoparticles at the nanoscale (typically between 1 and 100 nanometers), researchers have developed sophisticated drug delivery systems capable of delivering therapeutic agents directly to tumor sites while minimizing systemic toxicity. Nanoparticles can be designed to encapsulate drugs, protect them from degradation, and release them in a controlled manner, thereby improving the pharmacokinetic and pharmacodynamic profiles of chemotherapeutic agents. Additionally, the surface of nanoparticles can be functionalized with ligands that recognize and bind to specific receptors on cancer cells, enabling targeted drug delivery [7]. The use of nanoparticle systems for targeted cancer drug delivery presents a promising approach to overcoming many of the limitations of conventional therapies [9].
Recent advances in the field have led to improved efficacy and specificity in drug delivery, paving the way for innovative cancer treatments. Furthermore, nanoparticles can be engineered to deliver not only traditional chemotherapeutics but also biologics, immune modulators, and genetic materials, which may provide synergistic effects in overcoming drug resistance and improving patient outcomes. Thus, the use of nanoparticle systems in cancer therapy represents a transformative approach that has the potential to enhance the effectiveness and reduce the side effects associated with current treatment regimens [8-10]. This review aims to provide an in-depth analysis of the recent advancements in nanoparticle-based cancer drug delivery systems, highlighting the challenges that hinder their clinical application and the opportunities that lie ahead. The following sections will discuss the different types of nanoparticles used in cancer therapy, recent technological innovations, the biological and regulatory challenges faced, and the future directions of this rapidly evolving field.
Overview of Nanoparticle Systems in Cancer Treatment

Nanoparticle-based Drug Delivery systems (NDDSs) have emerged as a critical method for enhancing the targeting and therapeutic efficacy of anticancer agents. The small size of nanoparticles allows for the improved penetration of drugs into tumors and the potential for controlled release mechanisms. Various materials, including lipids, metals, and polymers, have been explored for their ability to deliver therapeutic agents effectively [7-12]. This (Figure 1) could illustrate various types of nanoparticles, including lipid-based nanoparticles (liposomes, SLNs, NLCs), polymeric nanoparticles (PLGA, PEG, chitosan), inorganic nanoparticles (gold, silver, silica), dendrimers, Carbon- Based Nanoparticles (fullerenes, CNTs, graphene oxide), protein based nanoparticles (albumin, gelatin), and micelles. Each nanoparticle type would be represented with a basic structure and a brief label describing its composition and unique properties (e.g., “Lipid-based: Encapsulates hydrophilic/lipophilic drugs; biodegradable,” “Inorganic: High stability, multifunctional,” etc.).
This figure helps visualize the structural differences and key characteristics of each nanoparticle type. Summarizing different types of nanoparticles used in cancer drug delivery, along with their key characteristics shown in (Table 1).
Types of Nanoparticles Used in Cancer Drug Delivery Lipid-Based Nanoparticles
Lipid-based nanoparticles, such as liposomes and Solid Lipid Nanoparticles (SLNs), are among the most widely studied nanocarriers for drug delivery. Liposomes are biocompatible, biodegradable, and capable of encapsulating both hydrophilic and hydrophobic drugs [13]. Recent advancements in liposomal formulations, such as Doxil (doxorubicin liposomes), have demonstrated enhanced circulation time and reduced cardiotoxicity in cancer patients [14]. SLNs provide additional advantages, such as improved stability and controlled drug release, making them suitable for cancer drug delivery applications.
Polymer-Based Nanoparticles
Polymeric nanoparticles, including micelles, dendrimers, and polymer-drug conjugates, offer versatile platforms for cancer drug delivery [15]. Polymeric nanoparticles, particularly those based on biodegradable Polymers Like Poly (lactic-coglycolic acid) (PLGA), have shown promise in delivering a wide range of therapeutic agents, including small molecules, proteins, and nucleic acids. These nanoparticles offer advantages such as controlled release, improved drug stability, and the ability to co-deliver multiple drugs [16]. PolyEthylene Glycol (PEG)- based micelles, for example, have been shown to increase drug solubility and prolong circulation time, reducing renal clearance and enhancing drug accumulation in tumor tissues through the Enhanced Permeability and Retention (EPR) effect. Dendrimers, due to their highly branched structure and multivalency, provide unique opportunities for targeted drug delivery and combination therapies [17].
Inorganic Nanoparticles
Inorganic nanoparticles, such as gold nanoparticles, silica nanoparticles, and magnetic nanoparticles, have gained significant attention due to their unique optical, magnetic, and electronic properties. These nanoparticles can be engineered for multimodal imaging and therapy, enabling simultaneous diagnosis and treatment-a concept known as theranostics. Gold nanoparticles, for example, can be used for photothermal therapy, where they convert light into heat to selectively kill cancer cells. Silica nanoparticles offer high drug loading capacity and tunable surface chemistry, making them suitable for multimodal imaging and drug delivery applications [18,19].
Stimuli-responsive nanoparticles
Recent research has focused on developing stimuliresponsive nanoparticles, which can release their therapeutic cargo in response to specific environmental triggers, such as pH, temperature, or enzymatic activity. These smart delivery systems offer the potential for on-demand drug release, improving the therapeutic index of anticancer agents. Additionally, the integration of targeting moieties, such as antibodies, peptides, or small molecules, has enabled the development of active targeting strategies, further enhancing the specificity and efficacy of nanoparticle-based drug delivery [20,21].
Challenges in the Development of Nanoparticle Systems for Cancer Drug Delivery
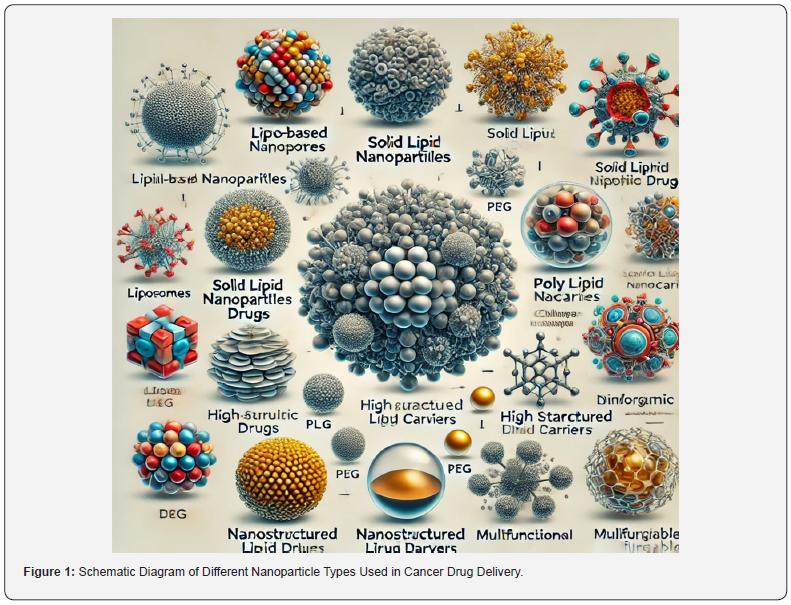
The development of nanoparticle systems for cancer drug delivery has shown significant promise, but translating these systems from bench to bedside remains a formidable challenge. Various biological, technological, regulatory, and manufacturing hurdles need to be overcome to realize the full potential of nanoparticle-based therapies in clinical settings. This (Figure 2) could depict the process of nanoparticle-mediated drug delivery in a cancer setting. The diagram would show a crosssection of a tumor and surrounding tissues, with nanoparticles in the bloodstream targeting and accumulating in the tumor site (enhanced permeability and retention effect). It could include different mechanisms such as passive targeting (via EPR effect), active targeting (via ligand-receptor interactions), and stimuliresponsive release (triggered by pH, enzymes, or temperature). Additionally, it could show intracellular drug release mechanisms, including endocytosis and drug release into the cytoplasm or nucleus. This figure demonstrates how nanoparticles enhance the specificity and efficacy of cancer treatment. Below, we discuss these challenges in detail.
Biological Challenges
The biological environment poses significant challenges to the development of nanoparticle-based drug delivery systems. The immune system can recognize and eliminate nanoparticles, reducing their circulation time and therapeutic efficacy. Moreover, the dense extracellular matrix in tumors can impede nanoparticle penetration, limiting drug delivery to cancer cells (Figure 2).
Immunogenicity and Clearance by the Immune System
One of the primary biological challenges facing nanoparticlebased drug delivery systems is the body’s immune response. The immune system can recognize nanoparticles as foreign invaders, leading to their rapid clearance from the bloodstream by the Mononuclear Phagocyte System (MPS), particularly by macrophages in the liver and spleen. This rapid clearance significantly reduces the circulation time of nanoparticles, limiting their ability to accumulate in tumor tissues [22,23].
To mitigate this issue, researchers have developed surface modification strategies, such as PEGylation, which involves coating nanoparticles with PolyEthylene Glycol (PEG) to create a hydrophilic barrier that reduces protein adsorption and recognition by immune cells. However, prolonged exposure to PEGylated nanoparticles can lead to the development of “anti- PEG antibodies,” which paradoxically accelerate clearance in subsequent administrations. This phenomenon, known as the accelerated blood clearance (ABC) effect, poses a significant challenge for the repeated dosing required in cancer therapy [22,23].
Tumor Microenvironment Barriers
The Tumor Microenvironment (TME) presents another significant biological challenge for nanoparticle drug delivery. The TME is characterized by abnormal vasculature, high interstitial fluid pressure, and a dense Extracellular Matrix (ECM), which collectively impede the penetration and distribution of nanoparticles within the tumor. The Enhanced Permeability and Retention (EPR) effect, which is often cited as a rationale for nanoparticle-based delivery, is highly variable among different tumor types and even within different regions of the same tumor. Moreover, the dense ECM and high interstitial fluid pressure can prevent nanoparticles from reaching the interior of solid tumors, limiting their therapeutic efficacy [24,25]. To overcome these barriers, researchers are exploring strategies such as designing smaller nanoparticles (<100 nm) that can better navigate the dense tumor stroma, and developing nanoparticles that can respond to specific tumor microenvironment cues, such as low pH or high enzyme concentration, to enhance penetration and release of therapeutic agents [24,25].
Heterogeneity of Cancer Cells
Cancer is not a homogeneous disease but is composed of a diverse population of cells with different genetic, phenotypic, and behavioral characteristics. This heterogeneity extends not only to the primary tumor but also to metastases, which may differ significantly from the primary site. Such diversity poses a significant challenge for targeted nanoparticle systems, as they may not effectively bind to all cancer cells or may only target a subset of the cell population, leading to incomplete treatment and potential relapse. To address this, there is a need for personalized medicine approaches where nanoparticles are tailored to target the specific molecular signatures of an individual patient’s tumor. However, this adds complexity to the development process and requires robust and rapid diagnostic tools to identify suitable targets [26].
Manufacturing and Scalability Challenges Reproducibility and Batch-to-Batch Consistency
The clinical translation of nanoparticle-based drug delivery systems requires scalable manufacturing processes that produce consistent and reproducible results. Unlike small molecule drugs, nanoparticles are complex, multi-component systems whose properties, such as size, shape, surface charge, and drug loading, can significantly affect their biological performance. Minor variations in the synthesis process can lead to batch-tobatch variability, which in turn can affect the safety, efficacy, and regulatory approval of the product. To ensure reproducibility, stringent quality control measures and robust manufacturing protocols are needed. Techniques such as high-throughput nanoparticle synthesis, real-time monitoring of nanoparticle characteristics, and the use of automated systems for scale-up are being developed. However, these approaches require significant investment and technological advancement, which can be a barrier for many researchers and companies.
Stability and Storage
Nanoparticles are prone to aggregation, degradation, and changes in physicochemical properties over time, which can affect their stability and efficacy. Stability is influenced by several factors, including temperature, pH, ionic strength, and the presence of other biomolecules. Ensuring the long-term stability of nanoparticles during storage and transportation is a critical challenge, as unstable nanoparticles may not perform as intended once administered to patients. Formulation strategies, such as lyophilization (freeze-drying) and the incorporation of stabilizing agents, are commonly employed to enhance nanoparticle stability. However, these methods can also introduce new challenges, such as the potential for drug degradation or the need for reconstitution before administration [27,28].
Regulatory and Safety Challenges Complex Regulatory Landscape
The regulatory landscape for nanoparticle-based therapeutics is complex and evolving. Nanoparticles do not fit neatly into existing regulatory categories, which were primarily designed for small molecule drugs or biologics. As a result, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) often require a more extensive set of data to assess the safety, efficacy, and quality of nanoparticle formulations. Key regulatory challenges include the lack of standardized guidelines for nanoparticle characterization, difficulties in demonstrating bioequivalence, and the need for long-term safety data. The potential for nanoparticles to cross biological barriers, such as the blood-brain barrier, raises additional safety concerns, as does their potential to accumulate in organs such as the liver, spleen, and kidneys, where they may exert toxic effects.
Toxicity and Off-Target Effects
While nanoparticles are designed to minimize off-target effects, their small size and unique properties can also lead to unexpected toxicity. For example, some inorganic nanoparticles, such as quantum dots or certain metal-based nanoparticles, can generate Reactive Oxygen Species (ROS) and induce oxidative stress, inflammation, or DNA damage. Similarly, the accumulation of nanoparticles in non-target organs can lead to organ-specific toxicity, posing a significant safety concern. To address these challenges, extensive preclinical studies are required to evaluate the toxicity, biodistribution, pharmacokinetics, and potential offtarget effects of nanoparticles. Additionally, there is a need for developing new in vitro and in vivo models that better mimic human biology to accurately predict the safety of nanoparticles before clinical trials [29,30].
Technological Challenges Drug Loading and Release Kinetics
Achieving optimal drug loading and controlled release kinetics is a major technological challenge in the design of nanoparticle drug delivery systems. High drug loading is desirable to reduce the number of nanoparticles needed to achieve a therapeutic effect, thereby minimizing potential toxicity. However, many nanoparticles have limited drug loading capacity, and high loading can sometimes compromise their stability or release profile. Controlled and sustained drug release is equally challenging to achieve. The release kinetics must be carefully tuned to ensure that the drug is released at the target site in the desired dose over a specified period. Uncontrolled release can lead to burst release, where a large amount of the drug is released quickly, potentially causing toxicity and reducing therapeutic efficacy. Researchers are exploring various strategies to address these challenges, such as designing stimuli-responsive nanoparticles that release drugs in response to specific triggers, such as pH changes, temperature, or enzymatic activity [29-32].
Complexity in Design and Functionalization
Designing nanoparticles with multiple functionalities, such as targeting ligands, imaging agents, and therapeutic payloads, adds significant complexity to their development. Each modification must be carefully optimized to maintain the stability and functionality of the nanoparticle while ensuring that the overall design remains biocompatible and non-toxic. Furthermore, the surface modification of nanoparticles to enhance targeting or evade immune detection can lead to unintended consequences, such as increased aggregation or altered biodistribution. Balancing these factors to develop a nanoparticle that is both effective and safe for clinical use is a significant technological challenge [29-32].
Opportunities Arising from Recent Research in the Development of Nanoparticle Systems for Cancer Drug Delivery
Recent advancements in nanotechnology and cancer biology have opened new avenues for the development of nanoparticlebased drug delivery systems, presenting numerous opportunities to enhance cancer treatment outcomes. These opportunities arise from the innovative design of nanoparticles, the discovery of new biomarkers and therapeutic targets, and the integration of advanced technologies such as artificial intelligence and personalized medicine [32]. Here, we explore the key opportunities stemming from recent research.
Precision and Personalized Medicin Personalized Nanoparticle Design
One of the most significant opportunities in nanoparticle-based cancer therapy is the ability to tailor nanoparticles to the specific needs of individual patients-a concept central to personalized medicine. Advances in genomics, proteomics, and cancer biology have led to the identification of a myriad of molecular targets specific to different cancer types and even subtypes within a patient. Nanoparticles can be customized to target these specific molecular markers, ensuring a more selective and effective delivery of therapeutics to cancer cells while sparing healthy tissues [10]. Recent research has focused on the development of nanoparticles functionalized with ligands, antibodies, or aptamers that specifically bind to receptors overexpressed on cancer cells. For example, nanoparticles conjugated with antibodies against the HER2 receptor have shown promise in targeting HER2- positive breast cancer cells, thereby providing a targeted delivery of chemotherapeutic agents specifically to the cancerous cells. This targeted approach can significantly reduce the side effects associated with conventional chemotherapy and improve patient outcomes [7-10].
Biomarker-Guided Therapy
The discovery of new cancer biomarkers provides an opportunity to develop nanoparticle systems that are responsive to specific biological cues. For instance, nanoparticles can be engineered to release their payload in response to enzymes, pH, or other conditions unique to the tumor microenvironment. Enzyme-responsive nanoparticles that degrade in the presence of Matrix MetalloProteinases (MMPs)-enzymes often overexpressed in the tumor microenvironment-represent a promising strategy for selective drug release [7,21]. Similarly, pH-responsive nanoparticles that release their cargo in the acidic environment typical of solid tumors offer another opportunity for precise drug delivery. These smart nanoparticles can enhance drug accumulation at the tumor site while minimizing systemic toxicity, providing a more effective treatment regimen for cancer patients [20,21].
Novel Nanoparticle Designs and Platforms Multifunctional and Hybrid Nanoparticles
Recent research has led to the development of multifunctional and hybrid nanoparticles that combine diagnostic and therapeutic capabilities; a concept known as theranostics. These nanoparticles can be designed to provide real-time imaging of tumor sites, enabling clinicians to monitor the distribution and efficacy of the therapeutic agent. For example, gold nanoparticles can be used for both imaging and photothermal therapy. When exposed to near-infrared light, these nanoparticles can generate localized heat, selectively destroying cancer cells while minimizing damage to surrounding healthy tissues [8,33]. The integration of multiple functionalities into a single nanoparticle platform not only improves therapeutic outcomes but also reduces the need for multiple agents, thereby simplifying treatment regimens and reducing potential toxicity. These advancements provide a significant opportunity to develop more efficient and effective cancer therapies [9,33].
Stimuli-Responsive Nanoparticles
The development of stimuli-responsive nanoparticles represents another promising area of research. These nanoparticles are designed to respond to specific stimuli-such as pH, temperature, redox conditions, or specific enzymes present in the tumor microenvironment-triggering the release of their payload only at the desired site of action. Such smart delivery systems offer the potential for on-demand drug release, enhancing the therapeutic index of anticancer agents [20]. For example, redox-responsive nanoparticles that release their drug payload in response to the high levels of glutathione found in cancer cells can provide a targeted treatment approach, minimizing exposure to healthy cells. This selective release mechanism reduces systemic toxicity and improves the efficacy of the therapeutic agent, providing a significant opportunity to improve patient outcomes [20,21].
Combination Therapy and Co-Delivery Nanoparticle-Mediated Combination Therapy
Nanoparticles offer a unique opportunity to co-deliver multiple therapeutic agents, enabling combination therapy that can target multiple pathways involved in cancer progression and resistance. By delivering a combination of chemotherapeutic drugs, nanoparticles can overcome Multidrug Resistance (MDR)-a significant challenge in cancer treatment. For instance, nanoparticles co-loaded with a chemotherapeutic agent and a MDR inhibitor can effectively bypass drug efflux pumps overexpressed in resistant cancer cells. Furthermore, nanoparticles can be used to deliver a combination of traditional chemotherapeutic drugs and novel agents such as Small Interfering RNA (siRNA), MicroRNA (miRNA), or CRISPR/Cas9 components for gene editing. This co-delivery approach provides an opportunity to simultaneously inhibit multiple targets, reduce the likelihood of resistance development, and improve the overall efficacy of cancer treatment [34-36].
Synergistic Effects of Combined Modality Treatments
Nanoparticles can also enable combined modality treatments, such as the simultaneous use of chemotherapy and radiation therapy or immunotherapy. For example, nanoparticles designed to release chemotherapeutic agents and radiosensitizers can enhance the effects of radiation therapy, leading to better tumor control and potentially lower radiation doses. Similarly, nanoparticles that deliver both a chemotherapy drug and an immunomodulatory agent can enhance the antitumor immune response, providing a synergistic effect that improves therapeutic outcomes [34-36].
Advances in Nanoparticle Characterization and Quality Control Improved Characterization Techniques
Recent advancements in nanoparticle characterization techniques have provided new opportunities to better understand and optimize the properties of nanoparticles for cancer drug delivery. Techniques such as high-resolution electron microscopy, Dynamic Light Scattering (DLS), Nanoparticle Tracking Analysis (NTA), and Atomic Force Microscopy (AFM) have enabled more precise measurement of nanoparticle size, shape, surface charge, and drug loading efficiency. These advancements allow for better control over nanoparticle properties, ensuring more consistent and predictable behavior in biological systems [37,38].
Enhanced Quality Control and Manufacturing
Improved methods for nanoparticle synthesis and quality control are critical for the clinical translation of nanoparticlebased therapies. Advances in microfluidic and automated nanoparticle synthesis technologies have enabled more scalable and reproducible production of nanoparticles with uniform size and shape, essential for maintaining batch-to-batch consistency. These technologies offer the potential for rapid, cost-effective production of high-quality nanoparticles, reducing the time and cost associated with bringing new nanoparticle-based therapies to market [37,38].
Integration with Emerging Technologies Artificial Intelligence and Machine Learning
The integration of Artificial Intelligence (AI) and Machine Learning (ML) with nanomedicine provides a significant opportunity to accelerate the development of nanoparticlebased drug delivery systems. AI and ML algorithms can be used to predict the behavior of nanoparticles in biological systems, optimize their design, and identify the most promising candidates for further development. These technologies can also help in analyzing large datasets generated from preclinical and clinical studies, identifying patterns and correlations that might not be apparent through traditional analysis methods.AIdriven models can also assist in the rapid screening of different nanoparticle formulations, significantly reducing the time required for preclinical testing. By incorporating AI and ML into the development process, researchers can enhance the precision, efficiency, and effectiveness of nanoparticle-based cancer therapies.
Integration with Immunotherapy
Recent research has highlighted the potential of combining nanoparticles with immunotherapy to enhance the immune system’s ability to recognize and destroy cancer cells. Nanoparticles can be designed to deliver immune checkpoint inhibitors, cytokines, or cancer vaccines directly to the tumor microenvironment, enhancing the antitumor immune response. Additionally, nanoparticles can be used to modulate the tumor microenvironment to make it more conducive to immune cell infiltration and activity, providing a promising strategy to overcome the limitations of current immunotherapies [8-39].
Regulatory Advancements and Market Potential Evolving Regulatory Landscape
The evolving regulatory landscape for nanoparticle-based therapies presents an opportunity to accelerate the clinical translation of these innovative treatments. Regulatory agencies such as the FDA and EMA are becoming more familiar with nanomedicine, leading to clearer guidelines and faster approval pathways for nanoparticle-based drug delivery systems. This evolving regulatory environment provides an opportunity for more rapid development and commercialization of nanoparticlebased therapies.
Expanding Market Opportunities
The market for nanoparticle-based cancer therapies is rapidly expanding, driven by the growing prevalence of cancer and the increasing demand for more effective and safer treatments. This market expansion presents significant opportunities for pharmaceutical companies and researchers to develop new nanoparticle-based therapies and capitalize on the growing demand for innovative cancer treatments.
Conclusion
The development of nanoparticle systems for cancer drug delivery holds significant promise for revolutionizing cancer therapy by enhancing treatment precision, efficacy, and safety. Recent advancements in nanoparticle design, such as multifunctional and stimuli-responsive nanoparticles, offer targeted delivery and controlled release of therapeutic agents, minimizing off-target effects and improving patient outcomes. The integration of nanoparticles with personalized medicine approaches enables tailored treatments based on individual tumor profiles, while combination therapies and co-delivery systems provide synergistic effects that can overcome drug resistance and improve therapeutic efficacy. Moreover, the integration of emerging technologies like artificial intelligence, machine learning, and immunotherapy with nanomedicine is creating new opportunities for optimizing nanoparticle systems, accelerating drug development, and enhancing the immune response against tumors. Improved characterization techniques and advances in scalable manufacturing processes further support the clinical translation of these innovative therapies. However, challenges such as immunogenicity, scalability, and regulatory hurdles remain. Addressing these challenges through interdisciplinary research and collaboration among scientists, clinicians, and regulatory bodies is crucial for the successful translation of nanoparticlebased cancer therapies from the laboratory to the clinic. As research progresses, the potential to significantly improve cancer treatment and patient outcomes through nanoparticle systems becomes increasingly attainable, positioning nanomedicine as a cornerstone of future oncological care.
Disclaimer
This manuscript reflects the views of the authors and should not be construed to represent views or policies of the affiliated institutions.
Conflict of Interest
The authors of this study declare that there is no conflict of interest in the publishing of this paper. There was no specific grant for this research from any public, commercial, or non-profit funding agency
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3): 209-249.
- Wang X, Zhang H, Chen X (2019) Drug resistance and combating drug resistance in cancer. Cancer Drug Resist 2(2): 141-160.
- Institute NC. Cancer Statistics - NCI. Natl Cancer Inst 2024.
- Cancer n.d (2024) https://www.who.int/news-room/fact-sheets/detail/cancer
- Abbas Z, Rehman S (2018) An Overview of Cancer Treatment Modalities. Neoplasm Intech Open.
- Bahrami B, Hojjat Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, et al. (2017) Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett 190: 64-83.
- Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, et al. (2020) Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front Mol Biosci 7: 193.
- Akabari AH, Patel S, Vaghela N, Ramani V, Shah DP (2023) Recent Application of Nanotechnology for Cancer Immunotherapy and Its Future Prospects. Int J Immunol Immunother 10(1): 69.
- Amreddy N, Babu A, Muralidharan R, Panneerselvam J, Srivastava A, et al. (2018) Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. Adv Cancer Re 137: 115-170.
- Nanotechnology Cancer Therapy and Treatment - NCI 2022. https://www.cancer.gov/nano/cancer-nanotechnology/treatment (accessed 1 September 2024).
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR (2016) Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res 33: 2373-2387.
- Shi J, Votruba AR, Farokhzad OC, Langer R (2010) Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett 10: 3223-3230.
- Samimi S, Maghsoudnia N, Eftekhari RB, Dorkoosh F (2018) Lipid-Based Nanoparticles for Drug Delivery Systems. Charact. Biol. Nanomater. Drug Deliv. Nanosci. Nanotechnol. Drug Deliv., Elsevier; 2018, p. 47-76.
- Pisano C, Cecere SC, Di Napoli M, Cavaliere C, Tambaro R, et al. (2013) Clinical Trials with Pegylated Liposomal Doxorubicin in the Treatment of Ovarian Cancer. J Drug Deliv 1-12.
- Xiao X, Teng F, Shi C, Chen J, Wu S, et al. (2022) Polymeric Nanoparticles-Promising carriers for cancer therapy. Front Bioeng Biotechnol 10: 1024143.
- Ambrogio MW, Toro González M, Keever TJ, McKnight TE, Davern SM (2020) Poly (lactic- co-glycolic acid) Nanoparticles as Delivery Systems for the Improved Administration of Radiotherapeutic Anticancer Agents. ACS Appl Nano Mater 3(11): 10565-10570.
- Gagliardi A, Giuliano E, Venkateswararao E, Fresta M, Bulotta S, et al. (2021) Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front Pharmacol 12: 601626.
- Wang F, Li C, Cheng J, Yuan Z (2016) Recent advances on inorganic nanoparticle-based cancer therapeutic agents. Int J Environ Res Public Health 13(12): 1182.
- Bhattacharyya S, Kudgus RA, Bhattacharya R, Mukherjee P (2011) Inorganic nanoparticles in cancer therapy. Pharm Res 28(2): 237-259.
- Gu M, Wang X, Toh TB, Chow EKH (2018) Applications of stimuli-responsive nanoscale drug delivery systems in translational research. Drug Discov Today 23(5): 1043-1052.
- Pham SH, Choi Y, Choi J (2020) Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics 12(7): 630.
- Pondman K, Le Gac S, Kishore U (2023) Nanoparticle-induced immune response: Health risk versus treatment opportunity. Immunobiology 228(2): 152317.
- Palmieri V, Caracciolo G (2022) Tuning the immune system by nanoparticle-biomolecular corona. Nanoscale Adv 4(16): 3300-3308.
- Ravi Kiran AVVV, Kusuma Kumari G, Krishnamurthy PT, Khaydarov RR (2021) Tumor microenvironment and nanotherapeutics: Intruding the tumor fort. Biomater Sci 9(23): 7667-7704.
- Zhou Y, Chen X, Cao J, Gao H (2020) Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J Mater Chem B 8(31): 6765-6781.
- Yang Q, Parker CL, McCallen JD, Lai SK (2015) Addressing challenges of heterogeneous tumor treatment through bispecific protein-mediated pretargeted drug delivery. J Control Release 220(Pt B): 715-726.
- Wu L, Zhang J, Watanabe W (2011) Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev 63(6): 456-469.
- “Stability Can be a Problem” — Dealing with Nanoparticle Stability Challenges in Drug Formulation n.d. https://oxfordglobal.com/formulation/resources/stability-can-be-a-problem-dealing-with-nanoparticle-stability-challenges-in-drug-formulation (accessed 1 September 2024).
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, et al. (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20(2): 101-124.
- Najahi-Missaoui W, Arnold RD, Cummings BS (2021) Safe nanoparticles: Are we there yet. Int J Mol Sci 22: 1–22.
- Seleci M, Ag Seleci D, Joncyzk R, Stahl F, Blume C, et al. (2016) Smart multifunctional nanoparticles in nanomedicine. BioNanoMaterials 17(1-2): 33-41.
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, et al. (2018) Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnology 16: 1-33.
- Yang S, Wallach M, Krishna A, Kurmasheva R, Sridhar S (2021) Recent Developments in Nanomedicine for Pediatric Cancer. J Clin Med 10(7): 1437.
- Gupta P, Neupane YR, Aqil M, Kohli K, Sultana Y (2023) Lipid-based nanoparticle-mediated combination therapy for breast cancer management: a comprehensive review. Drug Deliv Transl Res 13(11): 2739-2766.
- Xiao B, Ma L, Merlin D (2017) Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin Drug Deliv 14(1): 65-73.
- Gurunathan S, Kang MH, Qasim M, Kim JH (2018) Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int J Mol Sci 19(10): 3264.
- Tiwari H, Rai N, Singh S, Gupta P, Verma A, et al. (2023) Recent Advances in Nanomaterials-Based Targeted Drug Delivery for Preclinical Cancer Diagnosis and Therapeutics. Bioengineering 10(7): 760.
- Crist RM, Clogston JD, Stern ST, Dobrovolskaia MA (2024) Advancements in Nanoparticle Characterization. Methods Mol Biol 2789: 3-17.
- Laetsch TW, Dubois SG, Bender JG, Macy ME, Moreno L (2021) Opportunities and challenges in drug development for pediatric cancers. Cancer Discov 11(3): 545-559.