Modern Innovative in The Pharmacy and Medicine for Patients in the Republic of Kazakhstan
Satayeva LG1*, Shopabayeva AR, Myrzabayeva NA1, Sagantayeva S Kh2 and Zikeeva SK3
1Department of Integrated Management Systems and Economics in Health Care, National medical university of S.D. Asfendiyarov, Republic of Kazakhstan, Almaty
Candidate of pharmaceutical sciences, professor of a pharmacy, the Republic of Kazakhstan.
1Department of medical sciences, National medical university of S.D. Asfendiyarov, Republic of Kazakhstan
2Associate Professor of the Department of Foreign Languages, Kazakh Medical University of Continuing Education, the Republic of Kazakhstan
3Candidate of medical sciences, High School Healthcare, Almaty, Zhibek St. of a zhola 25
Submission: July 24, 2019; Published: September 17, 2019
*Corresponding author: Aditya Narula, Fellowship sports medicine abhinav bindra sports academy, India
How to cite this article: Satayeva LG, Shopabayeva AR, Myrzabayeva NA, Sagantayeva S Kh, Zikeeva SK. Modern Innovative in The Pharmacy and Medicine for Patients in the Republic of Kazakhstan. J Phy Fit Treatment & Sports. 2019; 6(5): 555698. DOI:10.19080/JPFMTS.2018.05.555698
Abstract
The problems of creating new medicines and providing them the population were considered according to the basic programs of the Government of the Republic of Kazakhstan, such as the “Strategy for Industrial Innovation Development of Kazakhstan for 2015-2020” , “Support for the development of the pharmaceutical industry of RK “, “Development and implementation of original Phyto preparations into manufacturing for the improvement of the pharmaceutical industry of Kazakhstan for 2015-2020yy.” and others. Based on the own researches, the authors analyzed the main innovative preparations for the treatment of patients with the socially significant diseases (mental diseases, tuberculosis, HIV infection and AIDS, diabetes mellitus and diabetes insipidus, oncological diseases, bronchial asthma, etc.) currently developed in the Republic of Kazakhstan. In the course of the researches, the main developers of the most innovative medicines were identified. Proprietary methodological approaches to solving the above-mentioned problems have been proposed. The main problems in the introduction of the new remedies into the pharmaceutical industry have been highlighted in this paper.
Keywords: Drug supply; The health system of the Republic of Kazakhstan; Regulatory framework; Equity and Availability of drug care; The role of the human factor; Patients with mental diseases of the Republic of Kazakhstan
Introduction
The choice of priorities in the scientific and technical sphere has a value that goes beyond the perspectives of its own development [1]. The use of a systematic approach in forming the innovation policy in many developed countries of the world is of high priority. The relevance of the study is that by the end of the XX century it became obvious that the level of the development of the scientific and technical sphere science, education, high-tech industries, world technology markets - defines the boundaries between the rich and poor countries, creates the basis for dynamic economic growth and becomes the most important factor in the formation of power centers.
Objective
i. -To analyze the possibilities of domestic pharmaceutical manufacturers in the production of medicines for treating patients with the socially significant diseases in the Republic of Kazakhstan
ii. -To analyze the update innovative inventions in the field of creating new medicines for significant diseases (mental diseases, tuberculosis, HIV infection and AIDS, diabetes mellitus and diabetes insipidus, oncological diseases, bronchial asthma, etc.)
iii. -To estimate the prospects in Kazakhstan’s own pharmaceutical industry for manufacturing the medicines for the treatment of this category of patients.
The level of innovative activity of update pharmaceutical enterprises in the Republic of Kazakhstan was estimated according to our specially developed questionnaire (consisting of 33 questions), which evaluated the ability of enterprises to manufacture medicines for the treatment of patients with the socially significant diseases, a content- analysis of documents regulating the innovative processes in the structure of the modern economy of Kazakhstan was conducted.
Method
Content -analysis
Results and discussion
Even an efficiently functioning market cannot compensate the inequalities in the amount of pharmaceutical care getting by different categories of patients. The concentration of the global economy makes companies to compete at ever higher levels of technology and at the same time stimulates the processes of specialization and localization of innovations. The efficacy of realizing the reforms of the pharmaceutical service is determined not only by the market laws, the regulatory and legislative base, but also by the scientific and methodological basis developed in accordance with the socially focused economic policy of the state. In Kazakhstan’s pharmaceutical practice, the new structures were formed rather spontaneously, being late and mainly in a chaotic way. They are poorly focused towards social interests; they do not have a stable legal base. The market mechanism is not able to meet the social requirements of accessibility, universality and social justice for medicinal assistance. The strategy of industrial-innovative development of Kazakhstan for 2015–2020, approved by the decree of the President of the Republic of Kazakhstan, and the Program for the Formation and Development of the National Innovation System until 2020, approved in April, 2015 by the Government of the Republic of Kazakhstan, were the main prerequisites on the implementation of timely diversification of the Kazakh economy.
Thus, the Republic of Kazakhstan took a course to create a competitive innovative economy. In the “Strategy of industrialinnovative development of Kazakhstan for 2015-2020yy.” it was noted that the state of health of the people determines the socio-economic, cultural and industrial development of any country. The public health sphere is one of the main and priority in the country in terms of sustainable and stable growth of the health state of population. The main task of public health in the framework of the Strategy is the creation and development of its own scientific and innovative potential, for which it is necessary to create conditions for the dynamic improvement of the health care system, considering the domestic and international experience. As noted, the state of health of the population of Kazakhstan, the sanitary-epidemiological situation and the development of the healthcare branch in the last decade were characterized by the both positive and negative indicators. In recent years, it has been possible to stabilize the main medical and demographic indicators as birth rate, mortality and average life expectancy; the incidence of infections caused by several pathogens has been reduced. Several reforms were undertaken in the healthcare sphere, some of which were successfully implemented, while others did not reach their logical conclusion. The latter include the creation of a regulatory legal base of the sphere, a significant increase in the financing of health care and medicinal supply [2,3]. A characteristic feature of the update period in the life of Kazakh society is the contradiction between the increase in the needs of patients with the socially significant diseases in medicines and the reduction in paying capacity to satisfy them.
Solving the problem of providing the population with medicines at the state level remains an important task, because the pharmaceutical care is one of the main components of the therapeutic process and preventive measures. The use of remedies is the most common and one of the most effective types of medical technology. Remedies are used for prophylaxis, treatment, reduction in the number of hospitalizations and length of staying in a hospital. Medicines are the unusual products. People do not choose the medicine themselves for taking, and it is not easy for them to decide how to replace one medicine with another. The level of expenditure on medicines to a greater or less extent remains outside the control of people, which is caused either by the nature of the disease or by the state of health. The cost of medicines might be high and often exceed the possibilities of people associated with payment, despite their undoubted and obviously significant role. Therefore, for all citizens it is important to have access to the necessary medicinal preparations. The market economy has put the pharmaceutical service of the Republic of Kazakhstan under the conditions of the need in reforming. The basis of the reforms in pharmacy was the search for a balance between the old, command and market economy with its needs in the field of providing the population with medicinal care. The model designed for operation under the conditions of a planned distribution system no longer works. Expectation of the omnipotence of the pharmaceutical market and the elimination of the state from the sphere of regulation of pharmaceutical care contradict the world experience of the postwar reforming in France, Germany, Japan and other countries and do not correspond to the real conditions of the economy.
Ways of transformation of the pharmaceutical service in the Republic of Kazakhstan cannot be considered in isolation from the changes occurring in the health systems of most countries at present. One of the main and responsible tasks of social policy throughout the world in the 1990s was the reduction of inequalities and injustices in social and health issues. Ensuring equity is one of the main issues of the European strategy of the world community, implemented by the World Health Organization (WHO) to achieve health for all people. In January 1998, at the 101st session of the WHO Executive Board, a decision was made concerning WHO’s activities in the field of medicines provision. It expresses concern about the current situation in the world, in which 1/3 of the world’s population does not have the guaranteed access to the most necessary medicines [4].
Four interconnected trends are observed in all parts of Europe: the changing role of the state and the pharmaceutical market; the decentralization of powers to the regional and / or municipal level of government; the engagement of the private sector; the change in the possibilities of citizens to exercise their rights to complete the reliable medicinal assistance. The main opportunity to ensure the equitable access to essential medicines for patients with the socially significant diseases is to increase the innovative activity of the domestic pharmaceutical enterprises. For forming the National Innovation System in the Republic of Kazakhstan, 4 main subsystems have been identified, where the state can effectively implement the innovation policy through the direct or indirect participation. As noted in the programs of the Government, in Kazakhstan a multi-level innovation infrastructure should be created, which is defined as a complex of interrelated manufacturing, consulting, educational and information structures. It should ensure the conditions for the implementation of innovation activities [2,5].
The experience of countries, including Finland, Singapore, South Korea, shows that government intervention in the innovation processes is necessary in order to create incentives for innovation that don’t generate sufficiently. Today, Kazakhstan has established the state development institutes, including the National Innovation Fund. Joint Stock Company “National Innovation Fund” was established by the decree of the Government of the Republic of Kazakhstan No. 502 of 30.05.03 “On the establishment of the joint stock company” National Innovation Fund “with 100% state participation in the authorized share capital. The authorized share capital of the fund is 150 million dollars. The purpose of the fund is to increase the overall innovative activity in the country, including the promotion to the development of high-tech and knowledgeintensive industries. At present, innovation is the key to survival; therefore, more and more pharmaceutical companies are considering them as components of success that can speed up the opening of the new commercial designs in the market. Application the external sources has become a powerful trend in the industry. As our analysis showed, despite there are few innovations in the pharmaceutical sector of the Republic of Kazakhstan, today we have the original domestic designs.
As noted by J.M. Arystanov and S.M. Adekenov, in case of successful use of innovations in Kazakhstana the second breakthrough in industry will happen. However, this time it happens not because of trade in minerals, but due to hightech industrial production [3]. According to Kazakh scientists’ opinions, today the scientific and industrial components of the pharmaceutical industry of the republic have reached such a level of development, when it is possible and necessary to talk about a systematic reduction of the country’s dependence on the import of medicines [2,3]. It is confirmed by the development and readiness for the industrial production of more than 20 original phytopreparations (arglabin, solkosollin, bialm, topolin, glycardine, etc.) by the Kazakhstan Institute of Phytochemistry.
Kazakhstan chemists and pharmacologists have got the great scientific potential, which allows them to create unique products that are competitive in quality and efficiency in both local and foreign markets [3, 5]. Thus, in Kazakhstan, the Government supported the production of the famous anti-tumor arglabin preparation, invented by Karaganda pharmacists of the Institute of Phytochemistry. Currently, this drug is included in the list of vitally essential medicinal preparations. Based on the Institute of Phytochemistry in Karaganda, the construction of a pharmaceutical plant with a production capacity of 2 million ampoules, 150 million tablets and 2 million packages of soft forms (ointment, suppositories) of arglabin per year [3] has started.
To date, the production of arglabin with a capacity of 2 million ampoules per year has already been launched. Studies by Russian scientists have confirmed that the treatment of all types of cancer using this drug increases the efficacy of treatment by up to 80%. At present, in addition to Kazakhstan, this product has been patented in 11 countries of the world, including the USA, Germany, Japan, France and China. Russian researchers have found that arglabin also provides antiviral effect, so the drug is also effective in the treatment of HIV infection patients. According to estimates of oncologists the need of Kazakhstan oncological clinics in this preparation accounts 3.7 million ampoules per year, and with the growth in the number of cancer patients in the country this figure increases [3]. In the Republic of Kazakhstan there are many opportunities for the industrial production of complex drugs for the treatment of patients with tuberculosis, viral hepatitis, oncological diseases, diabetes, circulatory system diseases [2,3]. The formation and development of an effective innovation system in the country is becoming an effective leverage for ensuring the diversification of the economy based on the application of knowledge and the introduction of innovations, and it has a huge impact on ensuring sustainable economic growth. On 24.07.11, the Government of the Republic of Kazakhstan approved the Republican Science and Technology program “Development and implementation in production of original Phyto preparations for the development of pharmaceutical industry in the Republic of Kazakhstan “ for 2015–2020.
Thus, in Kazakhstan, the development of medicinal preparations from herbal raw material was recognized as the most perspective scientific direction in the development of original medicines. In Republic of Kazakhstan the developers and manufacturers of original Phyto preparations with different pharmacological effects are the Institute of Phytochemistry of the Ministry of Education and Science of the Republic of Kazakhstan (Karaganda), Chimpharm JSC(Shymkent), The Production Cooperative “ Kyzylmay” (Almaty) and a number of other enterprises. All of them are the executors of this program. 40 phytopreparations which can be classified into three groups according to degree of reliability for manufacturing were planned by the executors of this governmental program for the period of 2015-2020 yy.
As can be seen from table 1, the first group includes preparations ready for industrial production:
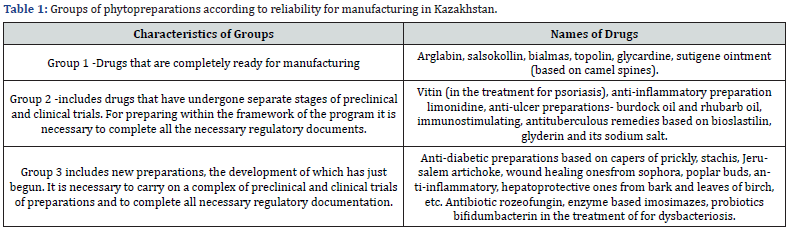
a) arglabin, salsokollin, bialmas, topolin, produced by the experienced pharmaceutical manufacturing by the Institute of Phytochemistry;
b) glycardine preparation, recommended for the treatment patients with chronic heart failure, was developed by the Institute of Chemical Sciences together with JSC “Chimpharm”.
c) asutigene ointment based on camel thorn was developed in the Al-Farabi Kazakh National Universityand its production was established at JSC “Chimpharm”.
d) The second group includes phytopreparations that have undergone separate stages of preclinical and clinical trials. For preparing the program it is necessary to complete all the necessary regulatory documents.
This group includes:
a) wound healing vitin preparation (recommended also in the treatment for psoriasis);
b) anti-inflammatory limonidin preparation;
c) anti-ulcer preparations as burdock oil and rhubarb oil;
d) immunostimulating, anti-TB ones based on bioslasticin, glyderin and its sodium salt.
As Prof. S.M. Adekenov noted, clinical trials of these drugs should be carried out considering all the requirements of the Ministry of Public Health of the Republic of Kazakhstan. The third group is composed of the new phytopreparations, the development of which has just begun. It is necessary to conduct a complex of preclinical and clinical trials of drugs and to complete all the necessary regulatory documentation.
Among the new phytopreparations being developed there are:
a) anti-diabetic ones based on capers of prickly, stachis, Jerusalem artichoke;
b) wound-healing products from sophora, poplar buds;
c) anti-inflammatory, adaptogenic, hepatoprotective ones from the bark and leaves of birch, etc.
Within the framework of the Republican scientific and technological targeted program “Use of methods of biotechnology and genetic engineering in medicine, agriculture and industry”, in Kazakhstan the biotechnological enterprises developed and organized the production of antibiotic rozeofungin, enzyme preparation imosimazy, probiotik bifidumbactinrin for dysbacteriosis treatment. In the South Kazakhstan State Medical Academy (Shymkent) a new combined preparation based on the root of licorice biaskin was developed. The proposed dosage form in the form of capsules has the following composition: bioslatin, ascorbic acid, starch. This composition includes two active substances - bioslatin and ascorbic acid, which determine hepatoprotective action, with intensive antioxidant activity. Kazakhstan researchers succeeded in obtaining immunoglobulins that are specific protein markers, typical for all tumor cells. On their basis, a preparation of normogen was created. It favorably differs from other oncological immunobiological preparations in that it has significantly fewer side effects, which were confirmed by the preclinical tests.
Rosezeostine preparation - ointment based on hydrophilic polyethylene oxide, containing anti-fungal polyene antibiotic roseophungin was created. The medicine is a highly active antifungal remedy. Based on the South Kazakhstan Pharmaceutical Academy, a preparation of glycyrrazide B6 was developed, which is positioned as a drug of the prolonged form of isoniazid on a dextran matrix, possessing the property of being selectively captured by macrophages, the ability to modulate the functional state of macrophages. This preparation gives a membrane-stabilizing effect, i.e, stabilizes the cell membranes with lysosomes, which contribute to decreasing the severity of the inflammatory process [2,3]. The advantage of this prescription is that it contains three active substances simultaneously, including bioslatilin - the final preparation of licorice root, the original hepatoprotection of plant origin containing Glycyrrhizinic acid.
Annually the scientific employees of the PC “Firm” Kyzylmai “develop and introduce into production 5-7 patented medical and prophylactic medicines on the basis of natural raw materials of herbs, berries and fruits, honey, wax and pollen, such as candles “Kyzylmai-lipofit”, “Kyzylmai with propolis”, “Kyzylmai with sea buckthorn oil”, “Kalefit”, “Metrophit” [2,3]. These preparations have been clinically tested in leading Kazakhstan and Russian medical centers. Their significant anti-inflammatory, antispasmodic, wound-healing and anti-septic action has been proved in the treatment for gynecological, proctological and urological diseases.
A new class of substances has been synthesized at the Institute of Chemical Sciences of the Republic of Kazakhstan - more than 10 compounds with anti-tuberculosis activity against resistant strains of tuberculosis, obtained in ‘in vitro’ studies on cell cultures, several compounds outperform rifampicin 20 times in the anti-tuberculosis activity of rifampicin. At the same time, they are less toxic than rifampicin. These results got positive results by the authoritative scientists of the Central Scientific Research Institute of Tuberculosis of the RAMS [2,3]. As noted in the Government’s programs, the economy of Kazakhstan should be developed through the introduction of domestic and foreign scientific and innovative designs, that implies a radical change in attitudes towards the development of science and innovation, education and training of professional stuff [3,5]. However, in the pharmaceutical industry of Kazakhstan there are also several problems associated with the introduction of new JICs [3,5].
The most important issue is to ensure the quality of domestic drugs, which is associated with the necessity for the development of an update system of standardization and certification. Firstly, it’s necessary to create a state bank of standard samples of biologically active substances that are active components of medicines. The problem of creating a bank of biologically active substances and data on them requires a solution at the governmental level. State certification and accreditation of land plots allocated for the cultivation of medicinal crops, especially endemic and acutely deficient species are required to ensure a stable raw material base of phytochemical production and stable quality of herbal raw materials, as well as rational and efficient organization of certification. Our analysis showed that at present time the Republic of Kazakhstan has its own innovative designs in the pharmaceutical industry. The Government of the Republic of Kazakhstan proclaimed that, as part of the development strategy, the state passes from passive investment to active policy and assumes the role of coordinator of investments for the development of new technologies in the Republic of Kazakhstan. For this purpose, a group of state financial development institutes is being created: the Kazakhstan Investment Fund, the Innovation Fund, the Export Credit and Investment Insurance Corporation. In addition, the Development Bank of Kazakhstan is being strengthened; centers for marketing and analytical research, engineering and technology transfer have been established [7].
In general, development institutes should form a unified system, the sustainable functioning of which will be based on the principles of decentralization, specialization, competition and transparency [2,5,6,8]. The Institute of Phytochemistry was offered to form a cross-cutting scientific and technical program for 2016-2018 on the development and organization of manufacturing the original export-focused Phyto preparations to develop the pharmaceutical cluster of the Republic of Kazakhstan.
Conclusion
i. Under the new economic conditions, the government of the Republic of Kazakhstan pays much attention to the innovative development of the economy, including the pharmaceutical healthcare sector.
ii. At present, the Republic of Kazakhstan has its own promising innovative medicine designs, which can be widely used in the clinical practice for the treatment of patients with many socially significant diseases.
iii. Nowadays the main innovators, which are forming an innovative development in the pharmaceutical industry of Kazakhstan, are the representatives of the Karaganda Institute of Phytochemistry.
iv. Management of medicinal provision of patients with the socially significant diseases under the new socio-economic conditions should be based on the formular system and the “medicinal standards” that are the part of the economic models of medical services.
v. Planning of medicinal provision should be done “from the bottom to up”: from planning in the health care institution to planning at the level of the national department of health care.
vi. To learn the basics of an effective system of compensation for expenses on buying the medicines by patients with the socially significant diseases the studying and summarizing of the experience of medicinal provision systems for citizens in countries with the developed economies are of great interest.
References
- Satayeva LG (2012) SWOT use the analysis for improvement of quality of provision of medicines of patients with diabetes and bronchial asthma in RK-Endocrinology Problems. Moscow 4(XII): 32-34.
- Satayeva LG (2017) Hepatoprotective Effect of Herbal Remedy with Anti-Tuberculosis Treatment-Journal Value in Health - ISPOR 20th Annual European Congress Research Abstracts.
- Satayeva LG (2017) Leadership in Nursing. - Journal Value in Health - ISPOR 20th Annual European Congress Research Abstracts. 20(9): A692.
- Satayeva LG (2017) Possibilities of Domestic Producers of the Republic of Kazakhstan in the Field of Manufacturing anti-tuberculosis Medicines. Journal Value in Health ISPOR 20(9)-A708.
- Decree of the Government of the Republic of Kazakhstan State Prize for the creation of new drugs Pharmacy of Kazakhstan 12: 3.
- WHO (1998) Focus on a patient? Pharmaceutical Sector Reform Strategy in the Newly Independent States. Action Program for Essential Medicines.
- Satayeva LG (2008) Mental disease and drug provision of patients in Kazakhstan Republic. Journal nevrology and psychatrii name Korsokova 108(8): 68-70.
- Satayeva LG (2017) Analysis of epidemiological data on burden disease in Europe Value in Health, Glasgow 20(9): 517.






























