Abstract
Herbal medicine is becoming more popular as a natural treatment for many diseases. Tecoma stans has historically been used in traditional medicine for several diseases. Methanolic Tecoma stans leaf extract was tested for its pharmacological properties. Initial phytochemical screening and GC-MS analysis found alkaloids, flavonoids, phenolics, terpenoids, tannins, sterols, saponins, glycosides, amino acids, and carbohydrates in the methanolic extract. Acute toxicity testing according to OECD guidelines 425 found the extract safe up to 2000mg/kg body weight. Researchers employed in vivo and in vitro models for DPPH free radical scavenging, protein denaturation, and cholinesterase inhibition (Ellman’s assay) to evaluate its pharmacological effects. In mice models of diazepam and aluminium chloride-induced amnesia, METS demonstrated significant antioxidant, mild anti-inflammatory and antibacterial, and excellent neuroprotective properties. Biochemical tests indicated increased Glutathione (GSH) and Superoxide Dismutase (SOD) and reduced brain Acetylcholinesterase (AChE) and oxidative stress markers (TBARS). Histopathological tests confirmed the extract’s neuroprotective effects. Tecoma stans methanolic leaf extract has antioxidant, anti-inflammatory, antibacterial, and anti-amnesic properties. These results suggest it might be utilised to make herb-based medicines.
Keywords: Tecoma Stans; Methanolic Extract; Pharmacological Activity; Antioxidant; Antimicrobial; Anti-Inflammatory; Neuroprotection; Acetylcholinesterase.
Abbreviations:GSH: Glutathione; SOD: Superoxide Dismutase; AChE: reduced brain Acetylcholinesterase; BAS: Baseline Activity Score; AD: Alzheimer’s Disease; APP: Amyloid Precursor Protein
Introduction
Herbal treatments have been used by individuals in the past as a complementary or alternative means of treatment to standard medical care. Only a few examples of the bioactive phytochemicals that may be found in plants include alkaloids, flavonoids, phenolics, tannins, terpenoids, and glycosides. This list is not exhaustive. There is a broad variety of pharmacological effects that these chemicals may perform, including those that assist decrease inflammation, kill germs, and protect neurones from injury [1,2]. Over the course of the last several decades, herbal formulations have become more popular as an alternative to synthetic pharmaceuticals for the treatment of memory loss and other neurodegenerative illnesses [3].
Cognitive Impairment and Pharmacological Targets
Amnesia, often known as memory loss, is a symptom that is associated with a number of neurological illnesses. A neurochemical imbalance, a brain injury, or exposure to medicinal medications such as aluminium salts and benzodiazepines are all potential scenarios that might lead to the development of this condition[4]. Inhibiting Acetylcholinesterase (AChE), reducing oxidative stress, and modulating neurotransmission are the primary emphases of therapy at the moment [5]. Nevertheless, it is of the utmost importance to discover alternatives to synthetic cholinesterase inhibitors such as donepezil and rivastigmine that are derived from plants and are somewhat less toxic [6].
Ethnopharmacological Relevance of Tecoma stans
The yellow trumpetbush, also known as Tecoma stans, is a plant that is used for medical purposes as well as ornamental purposes. It is a member of the Bignoniaceae family and is endemic to a broad region. According to the findings of ethnobotanical research [7,8], it has been historically used to cure a variety of conditions, including diabetes, gastrointestinal problems, bacterial and fungal infections, and inflammatory disorders. According to the findings of pharmacological research [9,10], extracts of Tecoma stans have properties that include anti-diabetic, antioxidant, antibacterial, hepatoprotective, and neuroprotective properties. Preliminary phytochemical research of Tecoma stans leaves have uncovered a number of chemicals, including alkaloids, flavonoids, tannins, terpenoids, and phenolic compounds. These compounds are all known for their antioxidant and neuroprotective activities. The methanolic extract has a concentration of these bioactive components that is particularly high. In view of the growing burden of cognitive impairment and the limitations of standard treatments, it is both important and expedient to explore the pharmacological activity of methanolic leaf extracts of Tecoma stans. This is because the pharmacological activity of these extracts is still being investigated.
Objectives
To assess the methanolic leaf extract of Tecoma stans’
phytochemical makeup and safety profile.
To examine the extract’s pharmacological activity in
experimental models, paying particular attention to its antiamnesic
properties.
Materials and Methods
Reagents and Chemicals
We purchased the following chemicals from SD Fine Chemicals Limited in Mumbai: methanol, chloroform, ethyl acetate, acetylcholine iodide, DTNB, disodium hydrogen phosphate, and sodium dihydrogen phosphate. These compounds were all obtained from the previous sentence. Donepezil, diazepam, and aluminium chloride were all provided by the laboratories located in Hyderabad, Hetero, and Mumbai, which are known as Ranbaxy and Himedia, respectively.
Plant Collection
The leaves of Tecoma stans were collected near Osmania University, Hyderabad
Preparation of Methanolic Extract
The dehydrated components of five kilogrammes of Tecoma stans leaves were subjected to soxhlation with methanol at room temperature for a period of five days. The resulting powder was then crushed into a coarse consistency. The crude methanol extract was subjected to partial fractionation, which was carried out with the assistance of solvents such as chloroform, methanol, and ethyl acetate. It was determined that the crude methanolic extract could be partitioned using a ratio of chloroform to water of 2:1. The water was fractionated once more using an 8:2 ratio of methanol to ethyl acetate in a separating funnel. This was done after the layers of chloroform and water had been separated. We drained and reused the methanol and ethyl acetate fractions that were produced as a consequence of evaporating them until they were completely liquid [11].
Initial Phytochemical Examination
The extract was subjected to primary phytochemical testing in order to ascertain the various chemicals that were discovered in the leaves of Tecoma stans [12].
Animals Used in Experiments
Swiss albino mice (20–25g) were supplied by Hyderabad Albino Study Laboratories. The study was authorised by the IAEC, GRCP, Bachupally, Hyderabad, India (Reg. No. 1175/PO/Re/S/08/ CPCSEA). All six animals lived in light-dark poly acrylic cages. Mice get regular food and water. One week before the trial, mice may customise the lab surroundings. All parties agreed to follow CPCSEA animal care and protection procedures.
Pharmacological Assessment in Vivo (Anti-Amnesic Activity)
Acute amnesia model produced by diazepam
An acute amnesic model induced by diazepam was utilised to test METS leaves’ anti-amnesic effects in vivo. This was done using Cook’s pole climbing gadget, Rotarod, and Actophotometer. Tables 1, 2, and 3 demonstrate the study’s design, and on the 8th and 9th days, researchers examined interaction limits such baseline activity score, fall off time, and passive avoidance time [13]. We carefully selected 30 male and female Swiss albino mice weighing 20–25 g and randomly allocated 6 mice to each of 5 groups.
Model of persistent amnesia caused by aluminium chloride
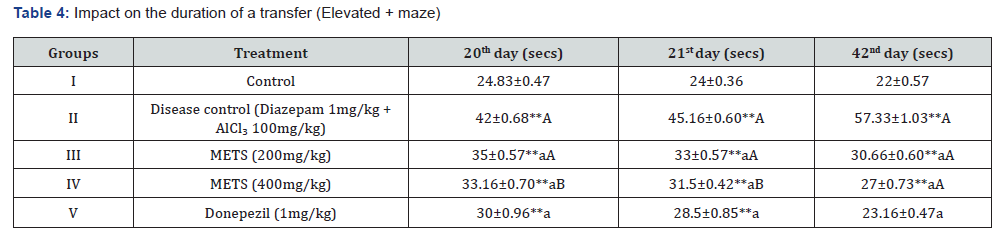
The methanolic extract of Tecoma stans was investigated for anti-amnesic effects in a model of aluminium chloride-induced chronic amnesia using Cook’s pole climbing apparatus and Elevated plus Maze [14]. Tables 4 and 5 demonstrate the research design, and behavioural characteristics such elevated plus maze transfer delay time and cook’s pole climbing apparatus pole climbing time were assessed on the 20th, 21st, and 42nd days. We divided 30 healthy male and female Swiss albino mice (20-25 g) into 5 groups of 6.
Biochemical Estimates
Mice were sacrificed by disarticulating their cervical spines after the experiment. Their brains were cautiously removed and put in a pH 7.4 phosphate buffer. After that, the brains were centrifuged for 15 minutes at 4500 rpm. The supernatants were utilised to determine AChE, TBARS, GSH, and SOD [15-18].
Ellman’s Method in-vitro AChE inhibition assessment
The Ellman reagent measured the plant extract’s acetyl cholinesterase inhibitory activity. In summary, 150 μL of 0.1M Na3 PO4 buffer (pH 8.0), 10μL of test extract (10-50μg/mL), and 20 μL of mouse brain homogenate enzyme solution (0.1 units/ mL) were combined and incubated at 25°C for 15 minutes. After introducing 10μL of DTNB (10mM), the substrate (10μL of 14 mM Acetyl thiocholine iodide) started the reaction. The enzyme cleavage of DTNB (5, 5-dithio-bis- [2-nitro benzoic acid]) with free thiocholine produces the coloured 5-thio-2-nitrobenzoate anion, which may be used to assess the hydrolysis of acetyl thiocholine iodide. The production of coloured compounds after 10 minutes was measured at 410 nm. The assay used the same technique for the test extract and utilised 10 μM Donepezil as a reference. The formula estimated AChE inhibition % [19].
Inhibition activity (%) = (1 − Absorbance of sample/ Absorbance of control) × 100.
Brain Tissue Histopathology
To extract the brains, one mouse from each of the five AlCl3 groups was killed after 42 days and had any superfluous skull connections severed. It was subsequently vertically split. Dissected hippocampi were stored in 10% formalin for histological studies.
Statistical Analysis
The data are shown as Mean ± SEM, with a sample size of 6. The groups were compared to control, disease control, and standard groups using Dunnett’s test. Notable findings were p < 0.001 and p < 0.05.
Results
Yield of Extraction
Soxhlation produced Tecoma stans leaf methanolic extract. The fraction yield was 33.3% and the percentage yield 24.54%.
Initial Screening for Phytochemicals
As part of the early study conducted on the methanolic extract of the leaves of Tecoma stans, a number of different compounds were discovered. These compounds included alkaloids, flavonoids, phenolics, sterols, terpenoids, tannins, saponins, glycosides, amino acids, and carbohydrates.
Studies on Acute Toxicity
Methanolic Tecoma stans leaf extract was evaluated at 2000mg/kg bd.wt on Swiss albino mice. The animal showed no toxicity or transience at 2000mg/kg bd.wt. The study found several physical and behavioural characteristics. Caloric intake and dietary consumption were also monitored. All animals were uninjured after 14 days of observation. Pharmacological testing was done orally at 200 and 400mg/kg body weight.
Anti-Amnesic Activity in Vivo
The diazepam-induced acute amnesic model was one of several METS anti-amnesic tests. Aluminium chloride-induced permanent amnesia model. An actophotometer, rotarod, and cook’s pole climbing apparatus measured Baseline Activity Score (BAS), fall off time, and passive avoidance time in diazepaminduced amnesia. Results were in Tables 1, 2, and 3. The elevated plus maze and cook’s pole climbing equipment were employed to measure passive avoidance and transfer delay time in aluminiuminduced amnesia. Tables 4 and 5 show these traits. Table 6 illustrates the aluminium chloride-induced amnesic model’s expected biochemical and behavioural characteristics. Ellman’s test measures Acetylcholinesterase (AChE), TBARS, GSH, and superoxide dismutase.
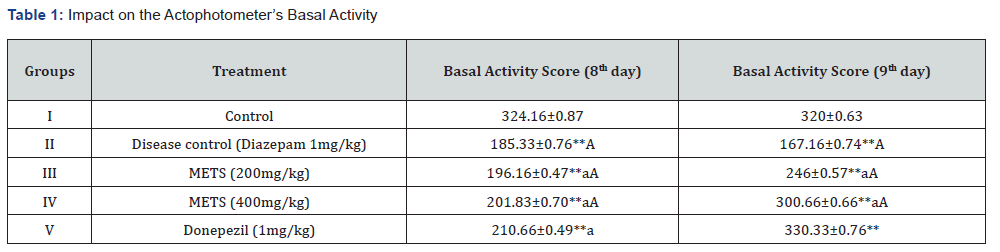
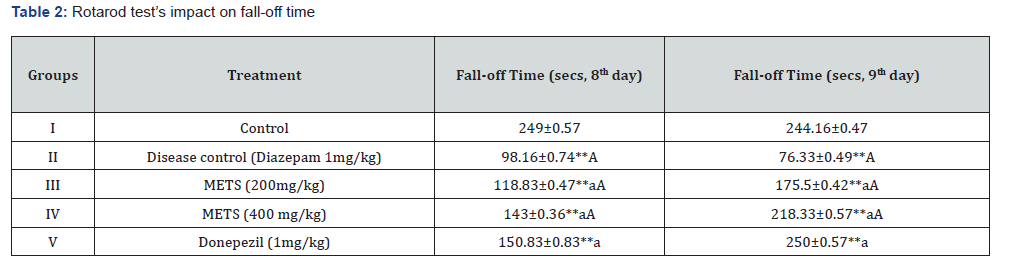
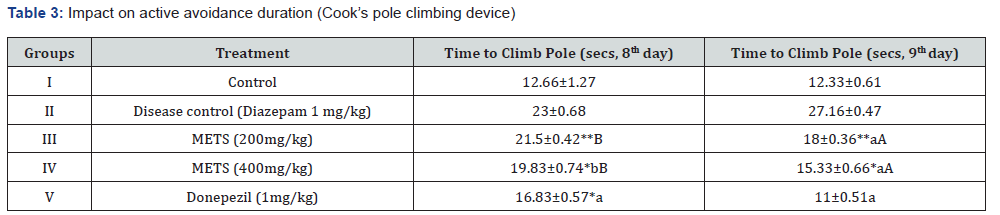
1. Diazepam-Induced Acute Amnesia Model: Actophotometerbased basal activity score, Rotarod-based fall off time, Cook’s pole climbing apparatus-based passive avoidance time
2. Chronic Amnesia Induced by Aluminium Chloride Model: Elevated plus Maze Transfer Latency Time, Cook’s Pole Climbing Device Passive Avoidance Time
Biochemical Approximations
Whole-brain AChE activity estimation
After then, Ellman’s test was used to measure biochemical parameters such AChE activity in mice’s brains during behavioural studies. The AlCl3-treated mice had considerably increased brain AChE activity than the control group. METS (200 and 400mg/ kg, bd.wt, p.o.) and Donepezil (1mg/kg, bd.wt, p.o.) significantly lowered AChE levels compared to the disease control group.
Total brain TBARS assessment
In biochemical markers such TBARS activity, AlCl3-treated animals had a substantial increase in brain TBARS levels compared to the control group. Compared to the disease control group, METS (200 and 400mg/kg, bd.wt. p.o.) and donepezil (1mg/kg) reduced TBARS levels.
Whole-brain GSH assessment
Mice brains were measured for biochemical GSH activity. The AlCl3-treated mice had significantly lower brain GSH levels than the control group. The METS (200 and 400mg/kg, bd.wt. p.o.) and donepezil (1mg/kg, bd.wt. p.o.) groups had greater GSH levels than the disease control group.
Assessment of whole brain SOD level
In mice, brain biochemical markers including SOD activity were examined. AlCl3-treated mice had considerably lower brain SOD activity than controls. METS (200 and 400mg/kg, bd.wt. p.o.) and Donepezil (mg/kg, bd.wt. p.o.) increased SOD significantly compared to the disease control group. Table 5
Ellman’s test for AChE inhibition in vitro
Table 7 compares the methanolic extract of Tecoma stans to donepezil in an in vitro AchE inhibition assay. The etiopathogenesis of Alzheimer’s disease is connected to acetylcholine deficit and Aβ production. METS’ anti-amnesic actions were evaluated by suppressing AChE. METS glycosides, tannins, terpenoids, alkaloids, flavonoids, phenolics, sterols, and saponins may have inhibited AChE. Donepezil, a brain-focused reversible acetyl cholinesterase inhibitor, is effective. Donepezil cures cognitive impairment by inhibiting cholinesterase.
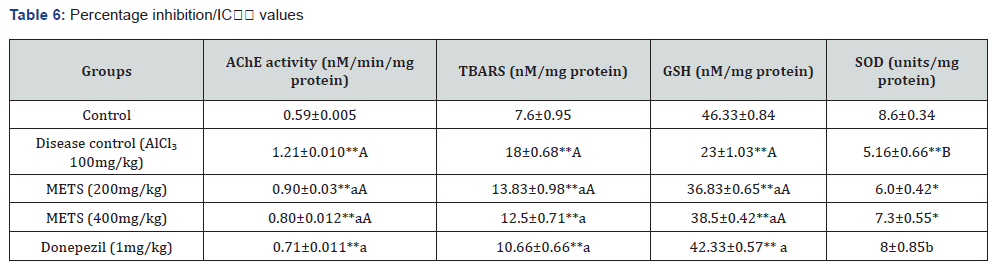
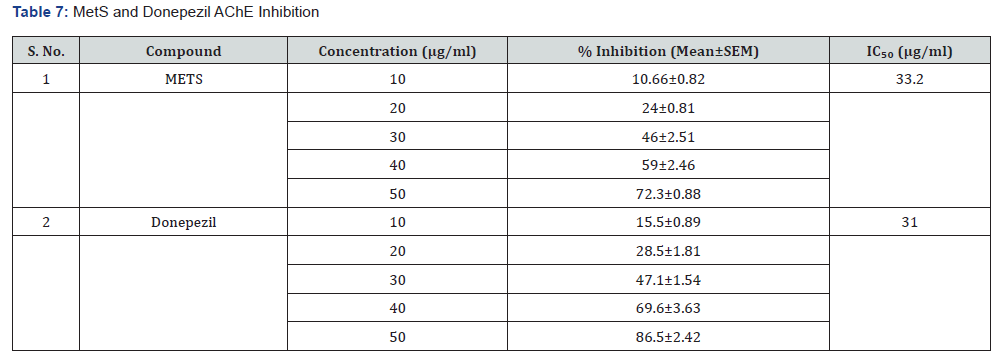
Histopathology observations
The purpose of this study was to investigate the histopathology of the hippocampus in mice that were given a model of chronic amnesia produced by aluminium chloride. The pathological change was seen under a light microscope after it had been stained with haematoxylin and eosin, as can be shown in Figure 1.
Control group: It was noted that the hippocampus has a densely packed 7–8 layer of pyramidal cells, each with its own conspicuous nucleus.
Disease control (AlCl3 100mg/kg, bd.wt, p.o): There were indicators of pyramidal cell loss and apoptotic cells in the hippocampus.
METS (200mg/kg, bd.wt, p.o): In the hippocampus, researchers found a pattern of dispersed pyramidal cells arranged in 2-3 layers, giving the area a faint apoptotic cell look.
METS (400mg/kg, bd.wt, p.o): The hippocampus is a highly organised structure of neural cells, having four or five layers of pyramidal cells.
Donepezil (1mg/kg, bd.wt, p.o): In the hippocampus, we found no apoptotic cells and well-organised neural cells with six to seven layers of pyramidal cells.
Discussion
In this work, animal models of amnesia caused by diazepam and aluminium chloride were used to investigate the possible anti-amnesic properties of a methanolic extract of Tecoma stans leaves (METS). A wide range of behavioural measures were investigated in this research. These measurements included the baseline activity score, fall-off latency, passive avoidance duration, and transfer delay for the participants. In addition, molecular markers such as Acetylcholinesterase (AChE), Thio barbituric acid reactive substances (TBARS), Reduced Glutathione (GSH), and Superoxide Dismutase (SOD) were examined in the brains of mice that had been rendered amnesic as a result of exposure to aluminium [20,21].
The phytochemical analysis of METS uncovered a diverse array of components, including alkaloids (tecomanine, tecostidine, tecostanine), indolic alkaloids (indole, tryptophan, tryptamine, anthranilic acid, skatole), other alkaloids (caffeine, boschniakine, 5β-hydroxyskitanthine, 4-noractinidine), flavonoids (luteolin, quercetin, chrysoeriol, flavones), phenolic compounds, sterols, terpenoids, tannins, saponins, iridoid glycosides, saponins, iridoid glycosides, and long-chain fatty acids. Furthermore, the presence of several bioactive molecules that have been associated with improved cognitive function was confirmed by GC-MS analysis [22]. These chemicals include alkaloids, flavonoids, phenolics, sterols, terpenoids, and fatty acids. The alkaloids that are naturally existing AChE inhibitors are the most promising candidates for the treatment of Alzheimer’s Disease (AD) because of their nitrogencontaining structures. There are many alkaloids that are naturally occurring. To be more specific, indole alkaloids are recognised as powerful natural AChE inhibitors. It is thought that the presence of alkyl substituents on the indole nitrogen might result in an increase in the affinity of the molecule for AChE [23,24].
As a result of polyphenols’ antioxidant and anti-amyloidogenic properties, including their ability to reduce Aβ aggregation, oxidative stress, and apoptosis, they have found widespread use in the treatment and management of cognitive impairments. Some instances of this include the capacity of quercetin to increase ApoE levels while simultaneously lowering concentrations of A in the cortex, and the ability of luteolin to reduce A production while simultaneously offering protection against cognitive decline. Furthermore, terpenoids exert control on the processing of Amyloid Precursor Protein (APP), so reducing the development of amyloid-beta (Aβ) and improving cognitive performance.
Irri did glycosides lessen the amount of damage done to the neurones in the hippocampus as well as memory deficits. They do this by boosting neuronal survival and repair. In addition, a number of studies conducted on animals that were affected by Alzheimer’s disease shown that omega-3 fatty acids, which include DHA and EPA, had the ability to enhance cognitive performance [25]. In the course of the investigation, the medicine donepezil served as a point of reference. The acetylcholinesterase inhibitor is a reversible acetylcholinesterase inhibitor that works centrally and increases cholinergic transmission by inhibiting the breakdown of acetylcholine at synaptic connections. This is accomplished by specifically decreasing AChE in the brain, which in turn improves cholinergic signalling and cognitive function.
Conclusion
The methanolic extract of Tecoma stans (METS) was shown to have a variety of bioactive phytoconstituents. These phytoconstituents include alkaloids, flavonoids, phenolics, sterols, terpenoids, tannins, saponins, glycosides, amino acids, and carbohydrates. The extract significantly improved cognitive performance in animal models with the help of its active components, particularly the alkaloids, flavonoids, and phenolics. This was due to the fact that these active components had complimentary effects. When compared to the medicine that is considered to be the gold standard, methylcholinesterase (AChE) activity was considerably suppressed by METS at both dose levels. This suggests that METS may have a role in cholinergic neurotransmission. In addition, histological examination of brain tissues obtained from mice that had been administered either METS or Donepezil confirmed the neuroprotective effect by repairing neuronal layers and retaining pyramidal cells in the hippocampus. When taken as a whole, these findings demonstrate that METS has potent anti-amnesic and neuroprotective capabilities, which bode well for its potential use as a therapy for cognitive impairment and Alzheimer’s disease.
References
- Patwardhan B , Vaidya AD, Chorghade M, Joshi SP (2015) Reverse pharmacology and systems approaches for drug discovery and development. Current Drug Discovery Technologies 11(2): 159-168.
- Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4: 177.
- Howes MJ, Perry E (2011) The role of phytochemicals in the treatment and prevention of dementia. Drugs & Aging 28(6): 439-468.
- Terry AV, Buccafusco JJ (2003) The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J Pharmacol Exp Ther 306(3): 821-827.
- Kumar A, Singh A (2015) A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol Rep 67(2): 195-203.
- Ferreira VTH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: Targeting the cholinergic system. Current Neuropharmacology 14(1): 101115.
- Goyal M, Nagori BP, Sasmal D (2013) Review on ethnomedicinal uses, pharmacological activity and phytochemical constituents of Tecoma stans (L.) Juss. ex Kunth. Pharmacognosy Reviews 7(14): 147-152.
- Khattab AR, El-Sayed HM, Hafez DA (2023) Pharmacognostic and pharmacological investigations on Tecoma stans Juss. ex Kunth: A comprehensive review. Journal of Herbmed Pharmacology 12(1): 25-34.
- Salem MZM, Ali HM, Shanhorey NAEl, Camacho LM, Salem AZM (2013) Antioxidant and antibacterial activities of Tecoma stans (L.) Juss ex Kunth against pathogenic bacteria. AJMR 7(5): 418-426.
- Ganga R, Sushma M, Gouthami K, Michaela N (2020) Anti-amnesic effect of methanolic leaf extract of Tecoma stans: An experimental study in rodents. J Young Pharm 12(2s): S91-S97.
- Hossain M, Hdhrami SAl, Weli A, Riyami QAl, Sabahi JAl (2014) Isolation, fractionation and identification of chemical constituents from the leaves crude extracts of Mentha piperita L grown in Sultanate of Oman. Asian Pac J Trop Biomed 4(1): 368-372.
- Kandelwal KR (2005) Practical Pharmacognosy Techniques and Experiments. 1st (Ed.), Pune: Nirali Prakashan 30-149.
- Ganga RM, Srilakshmi S (2018) Cognitive Enhancing, Anti-Acetylcholinesterase and Antioxidant Properties of Tagetes erecta against Diazepam Induced Amnesia in Rodents. Int J Pharm Sci Drug Res 10(6): 474-479.
- Ganga RM, Srilakshmi S (2018) Anti-Amnesic effect of methanolic extract of Tagetes erecta flower heads on Aluminium Induced Cognitive Impairment in Albino Mice. Res J Pharmacogn Phytochem 10(4): 299-303.
- Ellman G, Courtney KD, Andres V, Feather SRM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2): 88-95.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2): 351-358.
- Beutler E, Duron, Kefly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882-888.
- Misra HP, Fridovich I (1976) The oxidation of phenylhydrazine: Superoxide and mechanism. Biochem 15(3): 681-687.
- Singh V, Kahol A, Singh I, Saraf I, Shri R (2016) Evaluation of anti-amnesic effect of extracts of selected Ocimum species using in vitro and in vivo models. J Ethnopharmacol 193: 490-499.
- Wang H, Cheng H, Che Z, Huiling W (2016) Ameliorating effect of luteolin on memory impairment in an Alzheimer’s disease model. Mol Med Rep 13(5): 4215-4220.
- Yoo K, Park S (2012) Terpenoids as Potential Anti-Alzheimer’s disease therapeutics. Molecules 17(3): 3524-3538.
- Snowden S, Ebshiana AA, Hye A, An Y, Pletnikova O, et al. (2017) Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLOS Med 14(3):
- Meunier J, John I, Tangui M (2006) Antiamnesic and Neuroprotective Effects of Donepezil against Learning Impairments Induced in Mice by Exposure to Carbon Monoxide Gas. J Pharmacol Exp Ther 317(3): 1307-1319.
- Yeol KY, Young SP (2012) Terpenoids as Potential Anti-Alzheimer’s Disease Therapeutics. Molecules 17(3): 3524-3538.
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11(3): 315‐






























