Abstract
The Schiff bases (3a-f) with benzimidazole groups in ethanol were generated by refluxing Oct-2-ynoic acid (1,3-dihydrobenzimidazole-2-ylidene) amide with substituted amines. The synthetic Schiff bases’ geometrical geometries were confirmed by elemental analysis, FT-IR, UV-Visible, and NMR (‘H and ‘³C). The antibacterial activity of the compounds was evaluated using a microdilution technique. Eight strains of Gram-negative and six strains of Gram-positive human pathogenic bacteria, as well as seven strains of fungi (two species of Fusarium and five species of Aspergillus) were subjected to the chemical tests. The analysis revealed that Schiff bases had remarkable antibacterial action. The antibacterial efficacy against Klebsiella pneumoniae and Escherichia coli was greatest against compounds 3c-f, with a Minimum Inhibitory Concentration (MIC) of 7.8 μg/mL, surpassing that of the reference medicine nalidixic acid, which had a MIC range of 64-512 μg/mL. Based on the research, compound 3c is very effective against Aspergillus species and other fungal infections, with MFC values ranging from 7.8 to 15.6 μg/mL. We also evaluated Schiff bases for their ability to suppress the development of parasites and kill cells, in addition to our usual antibacterial testing. The ant plasmodial efficacy of compounds 3c and 3d was encouraging, with compound 3c being the most effective and least toxic to HeLa cells. The pharmacological investigation of Compound 3c, a Schiff base with broad-spectrum antibacterial activity, showed promise.
Keywords: Schiff Bases; Benzimidazole Derivatives; Antimicrobial Activity; Antibacterial Activity.
Abbreviations:MIC: Minimum Inhibitory Concentration; SAR: Structure-Activity Relationships; NMR: Nuclear Magnetic Resonance; MFCs: Minimum Fungicidal Concentrations; FTIR: Fourier Transform Infrared; MFC: minimum fungicidal concentration UV-Vis: Ultraviolet-Visible.
Introduction
Imines, another name for Schiff bases, are formed via condensation of a primary amine with an aldehyde or ketone. This process results in the formation of the characteristic azomethine (-C=N-) functional group. Hugo Schiff [1] made the discovery of these compounds in 1864, and ever since then, they have been the focus of much research due to the many applications that they may have in the fields of biology and industry. The structural diversity, simplicity of synthesis, and stability of these compounds would contribute significantly to the advancement of research in the fields of pharmaceutical and medicinal chemistry.
Pharmacological Significance
There is a broad spectrum of biological effects revealed by Schiff bases and their derivatives, such as antibacterial, antioxidant, anticancer, antimalarial, and anti-inflammatory activity [2,3]. Schiff bases that include heterocyclic moieties, such as benzimidazole, quinoline, or pyridine, have shown enhanced biological activity [4]. This is a result of their ability to interact with biomolecular targets. Furthermore, the presence of electron-donating or electron-withdrawing substituents inside the aromatic ring has the potential to further modify their potency via the mechanism of Structure-Activity Relationships (SAR).
Schiff Bases as Antimicrobial Agents
In light of the growing number of bacterial strains that are resistant to several antibiotics, there is an immediate and pressing need for antimicrobial medications that are both innovative and effective. Schiff bases, particularly those that are derived from heteroaromatic aldehydes and amines, have been shown to possess antibacterial and antifungal activities [5]. As a result of the azomethine linkage, they are able to attach themselves to enzymes and proteins that are found inside cells, which has the ability to disturb normal physiological processes and therefore stimulate them to take action [6]. Antimicrobial activity is improved by Schiff base-metal complexes due to the fact that these complexes are more lipophilic and are able to enter membranes with greater ease [7].
In light of the alarming rise in antibiotic resistance, there is still a lot of hope for the development of new Schiff bases with antibacterial properties. The Schiff bases derived from benzoimidazoles have attracted a lot of interest because of their well-known pharmacological properties and outstanding antibacterial actions. In this study, we will first identify Schiff bases containing benzimidazole groups; next, we will analyse and spectroscopically characterise these Schiff bases; and lastly, we will test their antibacterial activity against specific fungi and bacteria.
Objectives
To use spectroscopic and analytical methods to create
and describe a number of Schiff bases with benzimidazole moiety.
To assess the produced Schiff bases’ antibacterial
efficacy against certain bacterial and fungal strains.
Materials and Methods
All of the chemicals and reagents that were acquired from Sigma Aldrich in South Africa were not subjected to any extra purification procedures. These replacement amines were purchased from the same seller as the previous mentioned ones: p-aminobenzoic acid, 2-nitroaniline, 2-aminophenol, 2-aminopyridine, 2-aminophenolp- sulfonic acid, 2-trifluoromethoxyaniline, and 2-nitroaniline are all examples of compounds [8].
Schiff Base Synthesis (3a–f)
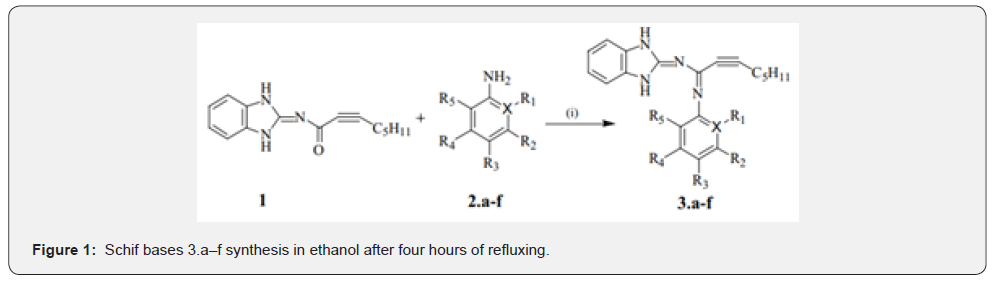
The Schiff bases were created by condensing equimolar amounts of Compound 1 (0.3921 mmol) and the corresponding substituted amines (0.3921 mmol) in hot ethanol. This was done in line with the procedure that was described earlier [9]. Immediately after the completion of four hours of refluxing, the components were strained out, and the solids that were obtained were washed with methanol, dried, and then placed in a desiccator. Alternatively, physical properties such as melting points, yields, and colours were recognised, and structures were confirmed using FTIR, UV-Vis, ~1H, and ~13C Nuclear Magnetic Resonance (NMR) spectra. To ensure purity, the elemental analysis was conducted.
Antibacterial Activity
The Schiff bases were produced by condensing 0.3921 mmol of Compound 1 and 0.3921 mmol of the corresponding substituted amines in hot ethanol. This process was carried out in order to manufacture Schiff bases. The procedures that were stated above were followed in order to carry out this [10]. After four hours of refluxing, the components were strained out, cleaned with methanol, dried, and finally deposited in a desiccator. The particles were then processed further. The last step was to dry them. Identification of various physical properties, including melting points, yields, and colours, and validation of structures via the use of Fourier Transform Infrared (FTIR), Ultraviolet-Visible (UVVis), Nuclear Magnetic Resonance (NMR), and Thirteen Carbon (13C) spectra, were all accomplished. To ensure the quality, the elemental analysis was carried out.
Antifungal Activity
The antifungal activity against numerous species of Aspergillus (A. flavus, A. niger, and A. fumigatus) and Fusarium (F. proliferatum, and F. verticillioides) was evaluated by the use of the broth dilution method. The test concentrations ranged from 500 to 7.8 μg/mL from beginning to end. Both nystatin and amphotericin B were used as standards. It was possible to determine the Minimum Fungicidal Concentrations (MFCs) by incubating the sample for a period of seventy-two hours.
Result and Discussion
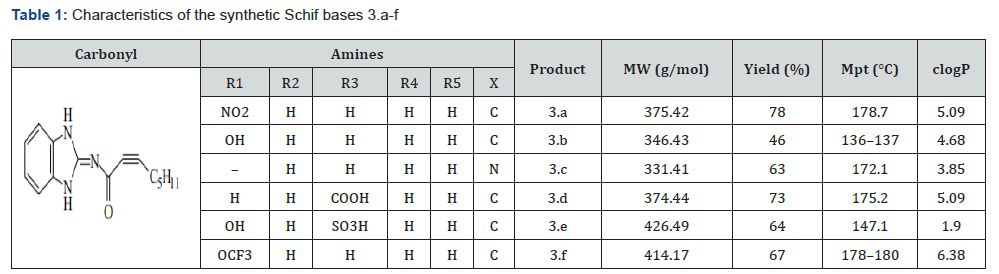
For the Schiff base compounds in the range of 3.a to 3.f, Figure 1 shows the process that was used. Compound 1, also known as Oct-2-ynoic acid (1,3-dihydrobenzoimidazole-2-ylidene) amide, was the first component used. To define and identify the structure of the produced ligands, measurements of spectral properties were used. Some of the tests that were conducted included the use of ultraviolet-visible absorption, Fourier Transform Infrared (FTIR), nuclear magnetic resonance proton and carbon (1 H and 13C NMR), and elemental analysis on carbon, hydrogen, and nitrogen. Table 1 displays the Schiff bases’ melting points and lipophilicity indices as calculated using Chem Draw Ultra 7.0. In addition, this table depicts the physicochemical properties of the Schiff bases.
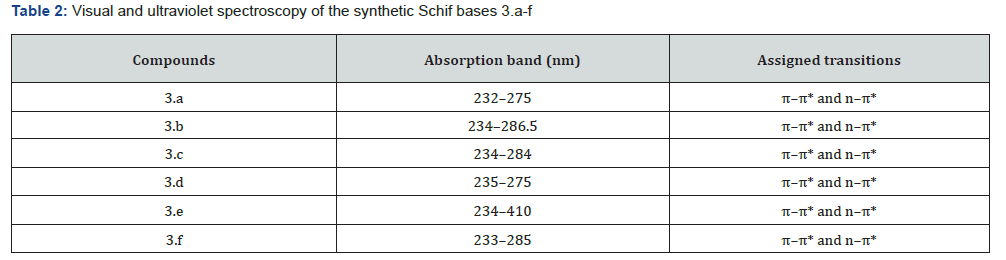
Electronic spectral
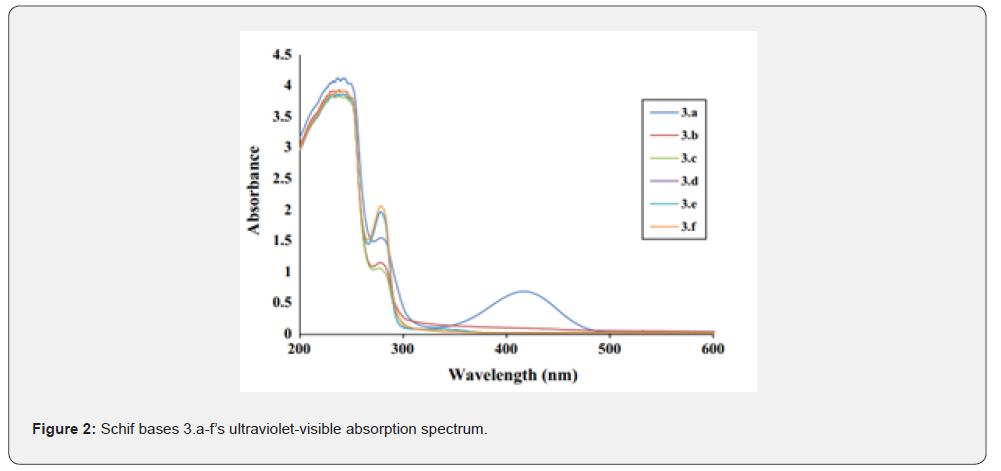
To create the desired compounds 3.a-f, Compound 1 (0.3921 mmol) and aniline derivatives (0.3921 mmol) were combined in a heated ethanoic solution and refluxed for four hours. The ligands’ UV-VIS absorption spectra were recorded in DMSO to explore their spectroscopic characteristics; Figure 2 shows the results. The normalised UV-VIS spectra of all the compounds (Table 2) show two absorption bands in the low UV region (232-290 nm), except for Compound 3.e, which has an extra band at 410 nm. The π-π* transition of the azomethine chromophore is thought to be caused by the Te bands seen below 250 nm. As mentioned in reference [11], the typical n-π* transitions of charge transfer between the C=N of the ligands are seen above 250 nm. The presence of a Te band at 410 nm in Compound 3e was caused by the distribution of free electrons in the aromatic ring, namely in the HSO3-position, as seen in Table 2. The results of the experiments supported this.
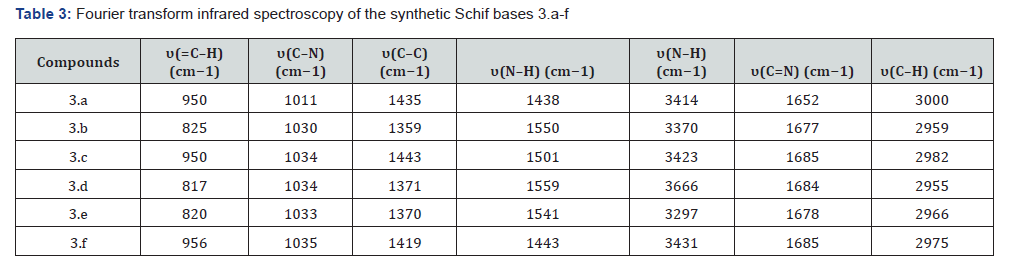
Fourier transform infrared
Table 3 shows that the Schif bases’ infrared spectra showed vibration signals at frequencies believed to be occupied by the relevant functional moieties and chromophores. Based on previous research [12], the ligands’ imine (C=N) interaction causes the stretching frequencies of Te to occur between 1652 and 1685 cm−1. Consistent with the results, these frequencies... The aromatic ring’s C-H stretching band corresponds to the lowintensity vibration signals detected at 2955-3000 cm−1. The aromatic ring was diagnosed by these signs. The sharp bands seen in the spectra of the compounds in the area of 3297-1500 cm−1 are caused by the N-H stretching frequencies of the imidazole moiety [13]. At a frequency of about 2231 cm−1, the C=O and S=O vibrations may be seen in compounds 3.d and 3.e. There is no evidence in the spectra that suggests the presence of the -NH2 group in the main amine, suggesting that the Schiff base ligands have been successfully synthesised.
NMR spectroscopy
Since the Schiff bases’ spectra did not show an imine proton, the condensation carbonyl was a ketone. The 1H NMR spectra of these substances revealed aromatic and aliphatic protons (Ar-H). Aromatic protons show single, doublet, and multiplet resonances at δ=6-7 ppm chemical shift from the aromatic group. Electronrich species on substituted rings induce proton chemical shift and deshielding. Compounds 3.a, 3.c, and 3.f exhibit extra Ar-H proton at δ=8 ppm due to this cause. Compound 3.b showed a chemical shift of 8.2 ppm, whereas Compound 3.e showed a shift of 10.10 ppm (δ), indicating the presence of phenolic protons as expected. At a chemical shift of 4 parts per million, the benzimidazole ring’s N-H signals are visible. If there is no contamination, the aliphatic side chain protons show as a doublet, triplet, or multiplet in the low chemical shift (0.8-2 ppm). C=N signals of the benzimidazole ring and imine of the synthesised Schiff bases were detected in Te 13C NMR spectra at δ=158-159 ppm. This matched forecasts. Aromatic carbons resonate at δ=108-140 parts per million, whereas alkyne carbons resonate at δ=70-80 parts per million. Between 13 and 30 parts per million, all aliphatic carbons were identified, with C‡C-CH2 resonating at roughly 30 parts per million. A ±0.6-unit discrepancy in recorded data confirmed that computed data matched the experimental percentage composition of C, H, and N in Schiff bases.
Pharmacological activity
Antibacterial activity
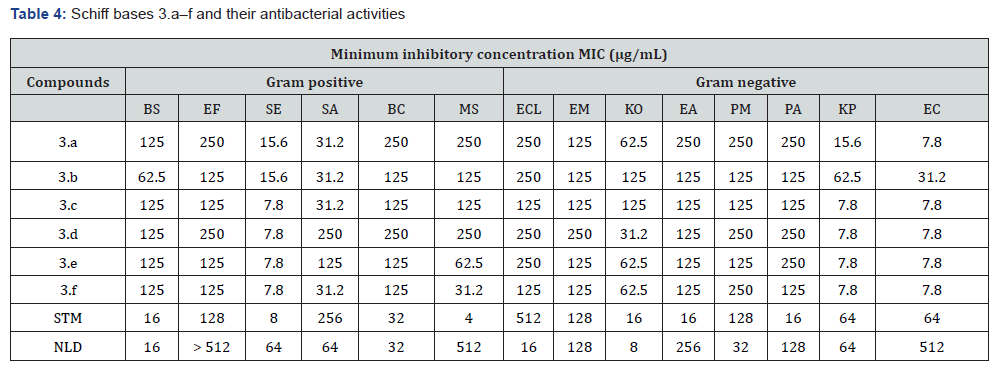
The antibacterial activity of Schiff base compounds (3.a-f) was examined in vitro against eight Gramme negative bacteria and six Gramme positive bacteria. The broth microdilution technique was used for this investigation. Using streptomycin and nalidixic acid as reference antibiotics, a comparison was undertaken between the Minimum Inhibitory Concentrations (MIC) of the compounds and those of the reference antibiotics. There is a display of the findings in Table 4. There were a variety of responses from the examined species to the ligand exposure, and their sensitivity varied depending on the concentration. The compounds that were examined exhibited antibacterial activity that ranged from good to high against a number of different bacterial representatives.
Among the Gramme positive bacteria that were examined, Staphylococcus epidermidis, for instance, had Minimum Inhibitory Concentration (MIC) values that varied from 7.8 to 15.6 μg/mL. Their effectiveness seemed to be 8.5 times more than that of nalidixic acid, which had a MIC of 64 μg/mL. Compounds 3.a-c and 3.f exhibited excellent antibacterial activity against Staphylococcus aureus, with a Minimum Inhibitory Concentration (MIC) of 31.2 μg/mL. This was in contrast to streptomycin, which had a MIC of 256 μg/mL. The compound 3.c-f had a higher Minimum Inhibitory Concentration (MIC) of 7.8 μg/mL against the Gram-negative bacteria Klebsiella pneumonia and Escherichia coli. This indicates that the compound exhibited superior antibacterial effectiveness in comparison to nalidixic acid, which had MICs of 64 and 512 μg/ mL against K. pneumonia and E. coli respective.
By virtue of the heterocyclic amine ring that it had, compound 3.c possessed the most extensive spectrum of activity among these compounds. In addition to the imine C=N bond, there are additional potential variables that might be responsible for the improved potency of these drugs. Both the benzimidazole ring and aromatic rings that have been substituted are present in these chemical compounds. The Gram-positive bacteria were able to neutralise the test chemicals more effectively than the Gramnegative bacteria. This was due to the variations in the makeup of the cell membranes between the two types of bacteria. It is also conceivable that the lack of activity might be attributed to the fact that the enzyme that is being targeted is not necessary or that there is inadequate interaction between the target and the targets [14]. It is possible that the variance in the components of the cell membrane is to blame. Positive Gramme bacteria are more vulnerable to attacks from the outside world because they lack the three extra components that are required for defence in Gramnegative bacteria. These components are lipopolysaccharides, phospholipids, and the periplasmic space [15]. There are a number of probable sources of bacterial resistance to the synthesised Schiff bases [16].
These include the enzymatic degradation of the synthetic compounds, changes to the bacterial protein that the compounds intend to target, and/or changes in the permeability of the membranes of the compounds that were tested. Furthermore, Gram-negative bacteria are very troublesome due to the fact that they are able to reverse the actions of almost all antimicrobial chemicals and treatments [17]. These molecules are responsible for the formation of the bilayer membrane that regulates and controls the flow of molecules inside the cell. Gram-negative bacteria may contain complex lipids that make it difficult for chemicals to diffuse into their cytoplasm, while Gram-positive organisms may not have this problem. They are able to survive chemical treatments more effectively than Gram-positive bacteria as a result of this.
Antifungal activity
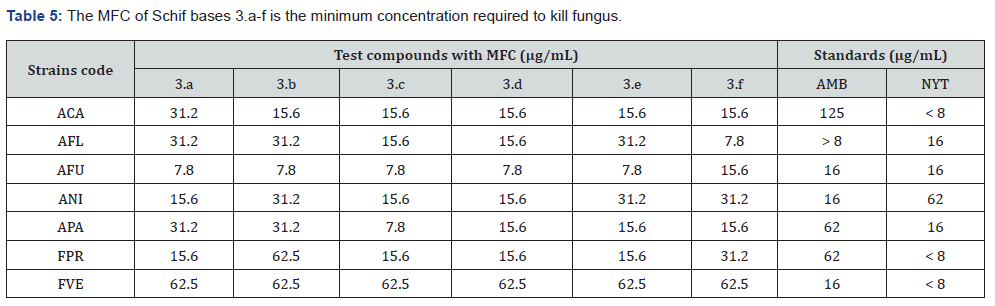
For the purpose of determining the antifungal power of the synthesised Schiff bases, we examined two different strains of Fusarium and five different species of Aspergillus. The results were compared with those of amphotericin B and nystatin, which were used as positive controls. The Minimum Fungicidal Concentration (MFC) was obtained using the broth dilution process, and the results are shown in Table 5. Across all of the strains that were evaluated, the antifungal effectiveness of these compounds ranged from moderate to high, and they performed much better than the antifungal drugs that are considered to be the gold standard. In comparison to amphotericin B, which had an antifungal activity of 125 μg/mL, the antifungal activity of all the substances that were tested was much greater (MFC 15.6 μg/mL) against Aspergillus carbonarius.
In light of this, it seems that amphotericin B is not their first recommendation. The test compounds had a significant cytotoxic impact against Aspergillus favus and A. carbonarius, as shown by their MFC values of 7.8 and 15.6 μg/mL, respectively with regard to the respective strains. Compound 3.c, which was among these compounds, had the most powerful fungicidal action, with a Minimum Inhibitory Concentration (MFC) that ranged from 7.8 to 15.6 μg/mL against the strains that were investigated. One possible explanation for this is because the molecule is hydrophobic and has a heterocyclic ring in its structure. It is simpler for chemicals that are made as well as those that occur naturally to interact with the cell membranes of microorganisms. This is because of the specific physicochemical features that each of these compounds have.
Chemicals and other molecules are regulated in their entry into the cytoplasm of a cell by the interaction of the components of the cell membrane with these molecules and chemicals. In the case of microbial cell membranes, molecules that are similar to one another will be pulled to the hydrophobic surface. This phenomenon was shown to be regulated by lipophilicity (clogP), which was identified as an important component controlling it. In spite of the widespread belief that the antimicrobial efficacy of Schif base series is directly proportional to the clogP value, the results of this study indicate that the activity of these Schif base series is determined not by the experimental lipophilicity values but rather by other parameters, such as the geometrical arrangement, stability, and polarity of the ligand. Compound 3.e had higher antibacterial activity in comparison to Compound 3.a, which included Te nitro (-NO2), both when the compound was in the ortho position and in comparison, to its analogue, fuorinate (-OCF3). Their counterpart, compound 3.b, which is a hydroxyl (-OH) compound, shown more effectiveness against fungus. The data shown in Table 5 demonstrates that the carboxyl and sulfonyl-based compounds (3.d and 3.e) were able to successfully inhibit all of the studied strains of fungus. However, only A. favus and A. niger were able to achieve an MFC concentration of 31.2 μg/mL throughout the experiment.
Conclusion
In order to effectively create and examine a collection of six Schiff base compounds with benzimidazole scaffolds, several methods such as ultraviolet-visible spectroscopy, Fourier transform infrared spectroscopy, elemental analysis, and Nuclear Magnetic Resonance (NMR) with 1H and 13C (NMR) were used. The antimicrobial test revealed that the compounds had a high level of efficiency against a broad variety of pathogenic bacteria, including Gram-positive and Gram-negative strains, as well as fungus. This was the conclusion reached by the researchers. It has been shown that the Schiff bases inhibit the growth of bacteria, which in turn may imply that they interfere with major metabolic processes. On the other hand, when the compounds were tested against HeLa cells, they exhibited a cytotoxicity that was very negligible. This suggests that they have the potential to be used in therapeutic settings without causing any harmful consequences. In light of all that has been taken into account, these Schiff bases have the potential to be ideal starting points for the creation of novel antibiotics.
References
- Singh K, Barwa MS, Tyagi P (2014) Schiff bases: Synthesis, characterization and biological evaluation. European Journal of Medicinal Chemistry 67: 519-530.
- Nair R , Patel, R , Baluja S (2019) Synthesis and antimicrobial evaluation of Schiff bases. Arabian Journal of Chemistry 12(4): 1234-1241.
- Patel R, Choudhary M (2020) Biological potential of Schiff bases and their complexes: A review. Journal of Molecular Structure 1203: 127416.
- Raman N, Pothiraj K , Muthuraj V (2012) Design, synthesis and antimicrobial activities of Schiff base transition metal complexes. Journal of Chemical Sciences 124(3): 637-651.
- Ali R, Shahid M, Khan A(2017) Biological activities of Schiff base metal complexes: A review. Journal of Coordination Chemistry 70(5): 935-964.
- Mishra A, Singh V (2016) Schiff bases: A review on biological activities. International Journal of Pharmaceutical Sciences and Research 7(2): 423-431.
- Khan S, Sharma R, Patel D (2018) Antimicrobial and structural studies of Schiff base metal complexes. Inorganic Chemistry Communications 93: 27-34.
- Wahe H, Asobo PF, Cherkasov RA, Augustin EN, Zacharias TF, et al. (2003) Heterocycles of biological importance: part 6. The formation of novel biologically active pyrimido[1,2-a]benzimidazoles from electron deficient alkynes and 2-aminobenzimidazoles. Arkivoc pp.170-177.
- Yousif E, Majeed A, Al-Sammarrae K, Bashar A, Jumat S, et al (2013) Metal complexes of Schiff base: preparation, characterization and antibacterial activity. Arab J Chem 10(S1): 1639-1644.
- CLSI (2008) Clinical, Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi 2nd CLSI Wayne 28(16).
- Selwin JR, Shiju C, Joseph J, Dhanaraj CJ , Arish D (2014) Synthesis and characterization of metal complexes of Schiff base ligand derived from imidazole-2-carboxaldehyde and 4-aminoantipyrine. Spectrochim Acta A Mol Biomol Spectrosc 133: 149-155.
- Kumaravel G, Raman N (2017) A treatise on benzimidazole based Schiff based metal(II) complexes accentuating their biological efficacy: spectroscopic evaluation of DNA interactions, DNA cleavage and antimicrobial screening. Mater Sci Eng C 70: 184-194.
- Batista RMF, Costa SPG, Belsley M, Raposo MMM (2007) Synthesis and second-order nonlinear optical properties of new chromophores containing benzimidazole, thiophene, and pyrrole heterocycles. Tetrahedron 63(39): 9842-9849.
- Mateus A, Gordon LJ, Wayne GJ, Helena A, Hanna A , et al. (2017) Prediction of intracellular exposure bridges the gap between target- and cell-based drug discovery. Proc Natl Acad Sci 114(30): E6231-E6239.
- Mai-Prochnow A, Clauson M, Hong J, Murphy AB (2016) Gram positive and Gram-negative bacteria differ in their sensitivity to cold plasma. Sci Rep 6: 38610.
- Dever LA, Dermody TS (1991) Mechanisms of bacterial resistance to antibiotics. Arch Intern Med 151(5): 886-895.
- Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. P T 40(4): 277-283.






























