Abstract
Pantoprazole, a benzimidazole proton pump inhibitor, was enteric coated to increase stability and efficacy. Direct compression produced pantoprazole sodium core pills. The substance included mannitol- and dicalcium phosphate-diluted microcrystalline cellulose. Disintegrant was croscarmellose sodium. Talc glided and magnesium stearate lubricated. Direct compression was selected over wet granulation because it needs fewer unit operations, energy, space, and labour, and lowers production costs. Both before and after compression, the compressed tablets’ hardness, weight fluctuation, friability, and drug content uniformity met standards. The enhanced core pills were dip-coated with enteric polymers including Eudragit L100 and cellulose acetate phthalate. After an acidic buffer (pH1.2), phosphate buffer (pH6.8) were used for in vitro dissolving tests. Formulation C2F9 excelled among all batches, with a hardness of 5.60±0.24 kg/cm², drug content of 99.08±0.35%, disintegration time of 7.02±0.21 min, and cumulative drug release starting at 120 min in acidic medium and reaching 99.72% within 180 min in phosphate buffer. After three months of testing at ambient temperature and 40°C/75% RH, the formulation was stable and effective.
Keywords: Pantoprazole Sodium; Proton Pump Inhibitor (PPI); Enteric-Coated Tablets; Direct Compression Method; Cellulose Acetate Phthalate (CAP); Eudragit L100; Dip Coating Technique; Dissolution Studies.
Abbreviations:PPI: Proton Pump Inhibitor; CAP: Cellulose Acetate Phthalate; GERD: Gastroesophageal Reflux Disease.
Introduction
Pantoprazole, which belongs to the class of drugs known as substituted benzimidazole, is a Proton Pump Inhibitor (PPI) that is very effective. GERD, Zollinger-Ellison syndrome, erosive esophagitis, and peptic ulcers are some of the disorders that may be helped by this medication. It works by permanently inhibiting the H⁺/K⁺-ATPase enzyme system in the parietal cells of the stomach, which in turn limits the amount of acid that is secreted by the stomach [1,2]. Pantoprazole has a limited oral bioavailability owing to its instability in acidic circumstances and the rapid destruction it undergoes in stomach pH [3]. This is despite the fact that it is useful in clinical settings.
Need for Enteric Coating
Pantoprazole requires formulation methods that can prevent its breakdown in the stomach and ensure its release in the small intestine, which is more acidic than the stomach. This is because pantoprazole is acid labile. This is achieved by the use of an enteric coating, which slows the release of the medicine in the stomach [4]. This coating makes it easier for the drug to be broken down and dissolved in the fluids of the intestinal tract. Melacrylic acid copolymers, such as Eudragit L100 and Cellulose Acetate Phthalate (CAP), are examples of enteric polymers that are often employed; the pH at which these compounds tend to dissolve is 5.5 [5]. A further example is the hydroxypropyl methylcellulose phthalate, sometimes known as HPMCP.
Formulation Approaches
When compared to wet granulation, direct compression is one of the several tablet manufacturing procedures that is widely used [6]. This is because it is straightforward, economical, and requires a smaller number of processing steps than wet granulation does. The production of pantoprazole tablets often involves the use of excipients such as croscarmellose sodium, which acts as a disintegrant; mannitol and dicalcium phosphate, which act as diluents; and magnesium stearate with talc, which functions as both a lubricant and a glidant [7]. Following the production of the core tablet, the enteric coating is typically applied by fluid-bed coating or dip coating processes, with polymers such as CAP and Eudragit L100 being used [8].
Evaluation Parameters
Enteric-coated pantoprazole tablets are tested for hardness, friability, weight variation, disintegration time, and drug content uniformity. After testing in simulated gastric fluid (pH 1.2), in vitro dissolution tests in phosphate buffer (pH 6.8) assess acid resistance and intestinal medication release [9]. Accelerated stability studies (40°C/75% RH) ensure formulation resistance during storage [10]. Pantoprazole enteric-coated tablets improve therapeutic effectiveness, patient compliance, manufacturing feasibility, and gastrointestinal stability. To manage gastrointestinal release, formulation characteristics such coating thickness, disintegration time, and polymer type must be optimized. This study designs and tests enteric-coated pantoprazole tablets coated with cellulose acetate phthalate and Eudragit L100 by direct compression and dip-coating.
Objectives
• To create pantoprazole sodium enteric-coated tablets by
the use of direct compression.
• To assess the physicochemical attributes, dissolving
profile, and stability of these tablets.
Materials and Methods
The following components are included in the formulation: magnesium stearate, microcrystalline cellulose, croscarmellose sodium, mannitol, dicalcium phosphate, and pantoprazole sodium. Talc, eudragit L-100, and cellulose acetate phthalate are some of the components that make up this product. Preparation of pantoprazole sodium tablets: In order to immediately punch an appropriate mix of granules into 200 mg tablets that included 40 mg of pantoprazole sodium sesquihydrate, a rotating tablet compression machine was used. Furthermore, a concave punch with a diameter of 8 mm was utilized throughout the process. For the purpose of maintaining their freshness, we collected all of the pantoprazole tablet batches and placed them in containers that were sealed [11,12]. Table 1
Compressed pantoprazole sodium tablet coating
Preparation of enteric coating solution: The straightforward solution method was used in order to produce a solution for the enteric coating. The formula asks for an enteric polymer (E1 and E2) or cellulose acetate phthalate (C1 and C2), a plasticizer (PEG 1.5 w/w), and a solvent (acetone or isopropyl acetone) with a weight-to-weight ratio of 6%. A paddle mechanical stirrer was used to aggressively agitate the mixture for one hour at a speed of one thousand revolutions per minute after the addition of diethyl phthalate and the subsequent adjustment of the volume with the leftover solvent mixture. Once again, the coating solution that was produced was filtered through muslin cloth [13]. Table 2
Enteric coating of pantoprazole sodium compressed tablets by dipping method: Coating the crushed tablets with an enteric coating polymer solution, such as Eudragit L100 or cellulose acetate phthalate, was accomplished by the use of the dipping technique. Both the anticipated increase in weight and the preservation of the tablet covering was successfully attained. According to the findings of the research conducted on coated tablets, measurements were taken of their thickness, weight fluctuation, and in vitro dissolution, in addition to the uniformity of their drug content [14,15].
Physicochemical evaluation of coating films: Polymeric films were made from the same polymer solution for thickness and solubility testing. A glass plate was covered with acetone and PEG to form polymeric films. A unique cover reduced solvent evaporation while drying the film at ambient temperature for 24 hours to achieve consistent smoothness. After cutting it into 1 cm2 pieces, the polymeric film was tested for thickness and solubility [16–18]. We measured completed film thickness using a thickness digital micrometre. The film’s solubility was tested at pH 1.2 and 6.8. The 1x1 cm2 coated film was weighed and put in a beaker with 20 mL of the appropriate pH medium before testing for solubility. The mixture was agitated for 1 hour at 37±1°C using a magnetic stirrer.
In vitro drug release studies: In vitro medication release from various formulations was examined using a USP dissolving device type II. For 900 mL and 2 hours, phosphate buffers with pH 6.8 and acidic buffers with pH 1.2 were utilized as dissolving media. The basket held the tablet. The temperature was maintained at 37±0.5°C and the stirring rate was 100 rpm. Samples were changed with same-volume dissolving media at intervals. Using a blank as a reference, a UV spectrophotometer evaluated the samples at 283 and 288 nm (pH1.2 and 6.8). Three release trials were done and the means shown versus time [19].
Stability studies: When performing stability investigations, the requirements established by the ICH were adhered to. The Pantoprazole sodium tablet formulations that were selected were stored under an aluminium foil cover for a period of three months. The temperature was maintained at 40±2 degrees Celsius, and the relative humidity was maintained at 75±5%. In order to conduct the research that we want to do at certain times; we extracted samples from each formulation. A physical appearance, the level of hardness, and the amount of drug content were evaluated in the samples that were removed [20].
Result And Discussion
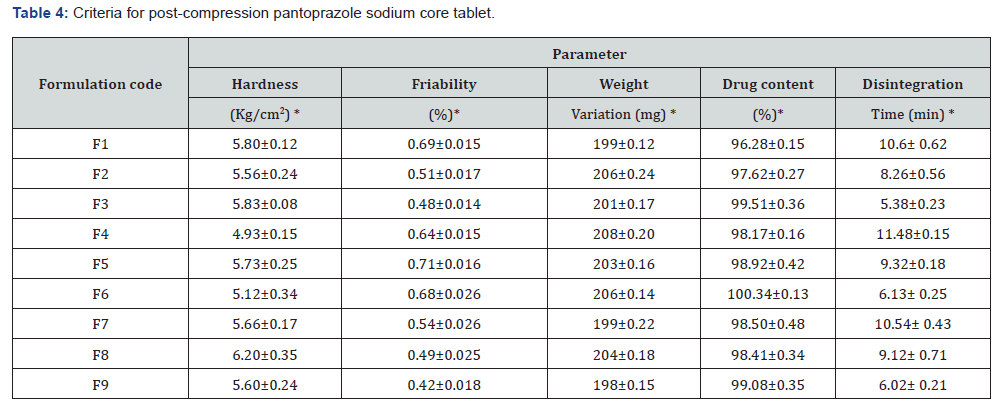
Direct compression was the method that was used in order to produce the pantoprazole powder mixture that was intended for tabletting. Based on the results shown in Table 3, the pantoprazole powder mixture that was manufactured underwent tests to determine its compressibility index, bulk density, angle of repose, and Hausner’s ratio. While the bulk densities of the granules were found to be between 0.306±0.03 and 0.384±0.04 gm/mL, the densities of the granules that were tapped ranged from 0.313±0.04 to 0.429±0.05 gm/mL. Through the determination of the granules’ angle of repose and Carr’s Index, we were able to assess the flow parameters of the aggregates. Compressibility measurements that fall within the range of 5.74±0.13 to 10.48±0.20 percent show that the flowability characteristics are adequate. All of the formulations had an angle of repose that was less than 30 degrees, which indicated that the manufactured granules had an acceptable flowability. The angles of repose ranged from 25.79±0.24 to 29.52±0.14.
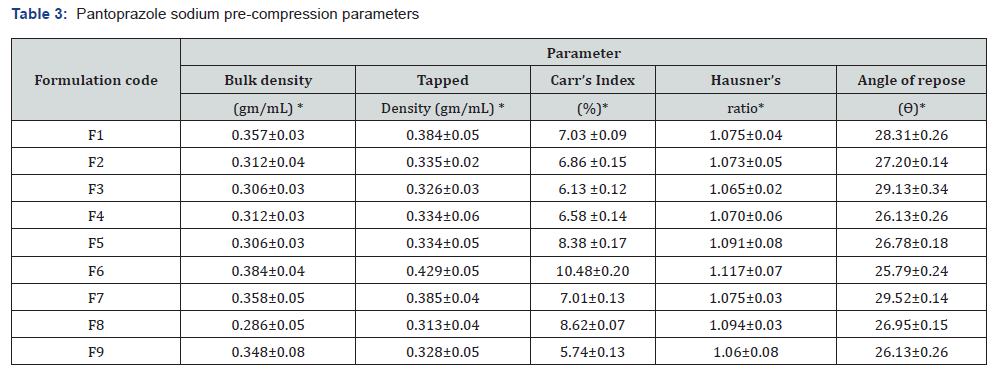
Post compression parameters of pantoprazole sodium core tablet: After being made utilizing the direct compression method, the pantoprazole tablets were put through a series of tests to determine their in vitro drug release, hardness, weight fluctuation, content uniformity, friability, and hardness (Table 4). In order for the product to be able to survive handling without breaking or crumbling, it is essential to manage its hardness. However, the product should not be so hard that it takes an excessive amount of time to disintegrate. A determination was made that the tablets had an average hardness that ranged from 4.93±0.15 to 6.20±0.35 kilograms per square centimetre. Tablet friability levels, which are impacted by hardness values, are often found to fall within the range of 1%. This is a frequent practice. It was established that the tablets that were created had a friability that was lower than 1% weight-to-weight. Pantoprazole sodium had a drug content uniformity that ranged from 96.28±0.15 to 100.34±0.13% when it was administered in tablet form. An average weight range of 198±0.15 to 206±0.24 mg was recorded out of the total number of individuals. Considering that the disintegration time varied from 11.48±0.15 to 5.38±0.23, it can be concluded that all of the findings are positive.
Physicochemical evaluation of coating films: The research conducted on Eudragit L100 and cellulose acetate phthalate looked at a number of different physicochemical properties, including film thickness, film weight, and film solubility, among other potential qualities. According to the findings shown in Table 5, the enteric polymer Eudragit L100, which is made up of cellulose acetate phthalate, was found to be completely soluble at a pH of 6.8 and insoluble at a pH of 1.2.
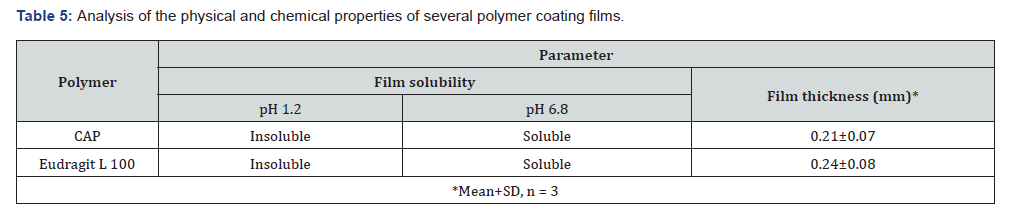
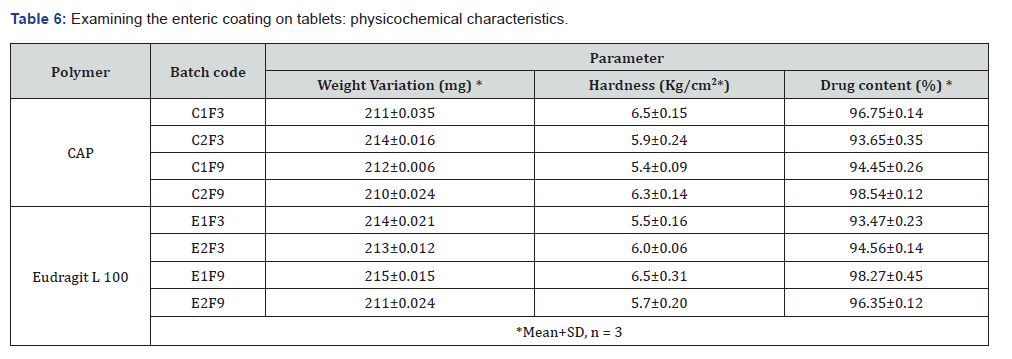
Physicochemical evaluation of pantoprazole sodium enteric coated tablets: With the best disintegration and drug content requirements, tablets F3 and F9 that have been coated using the dip coating procedure are the ones that have been coated. Table 6 contains the findings of the physicochemical analysis that was performed on the coated tablets that were used in the production process. Based on the findings, it was concluded that the range of weight fluctuation was between 214±0.021 mg and 0.211±0.024 percent. When determining the drug content, it was found that the range ranged from 93.47±0.23% to 98.45±0.12%. The hardness that was tested went from 5.2±0.11 to 6.5±0.15 kilograms per square centimetre.
In vitro drug release studies of enteric coated tablets: To determine the in vitro release of pantoprazole sodium, the produced tablets were put through a series of tests in two distinct buffer solutions.
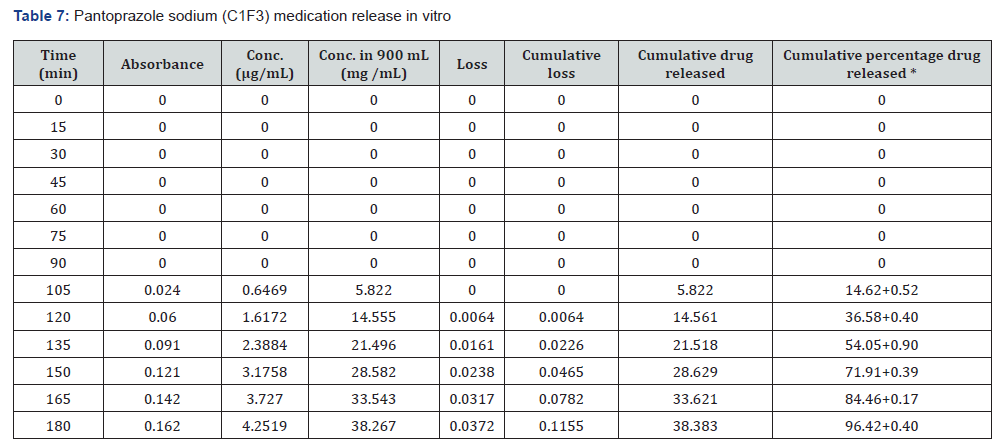
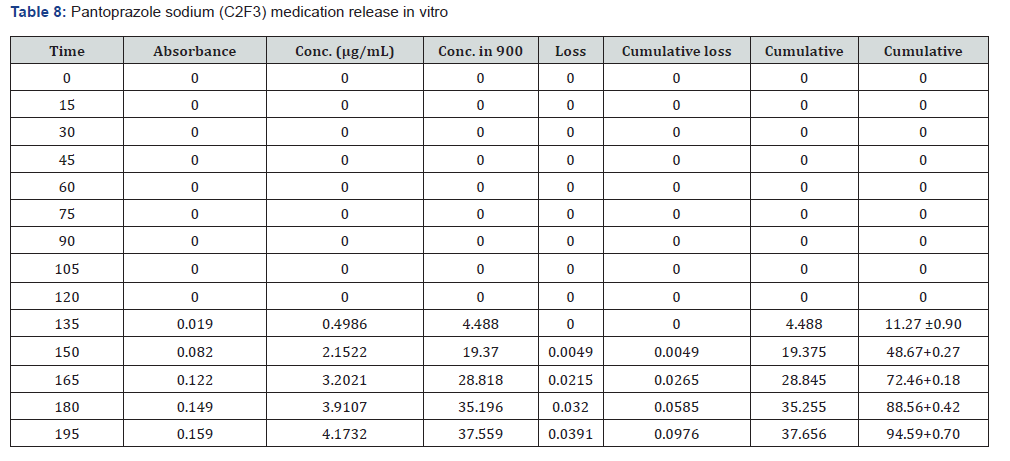
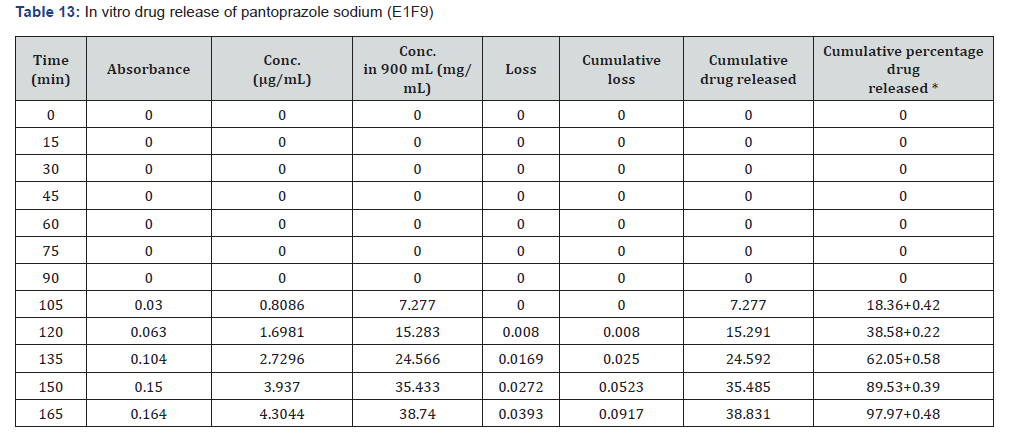
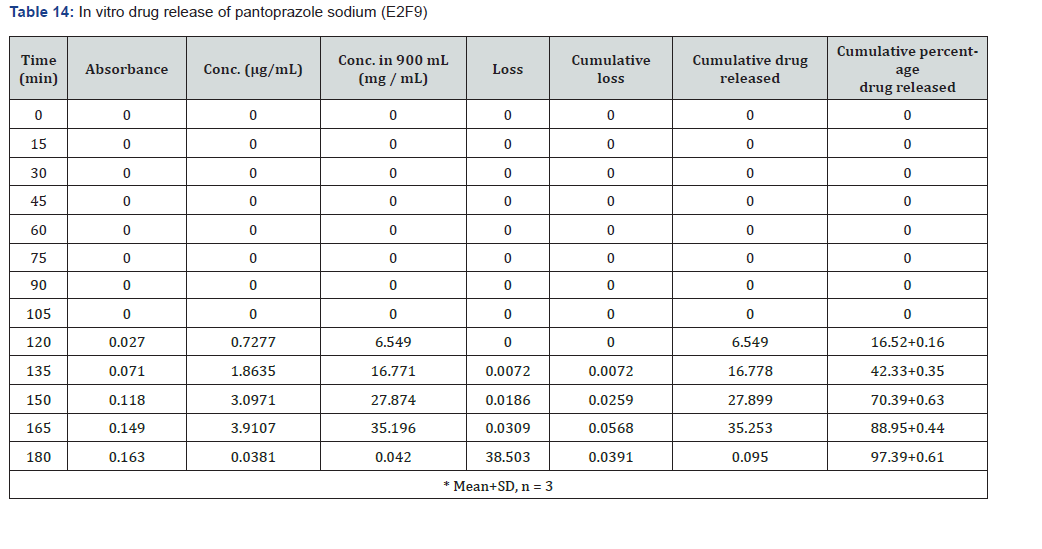
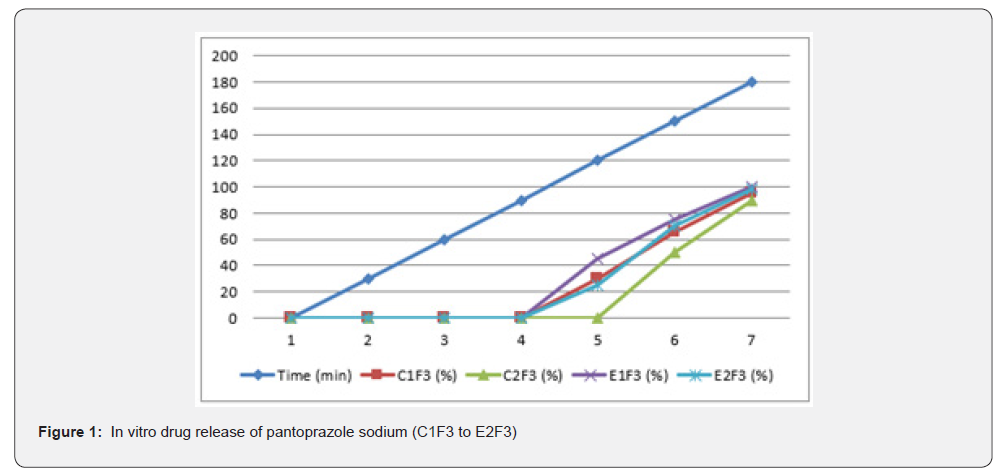
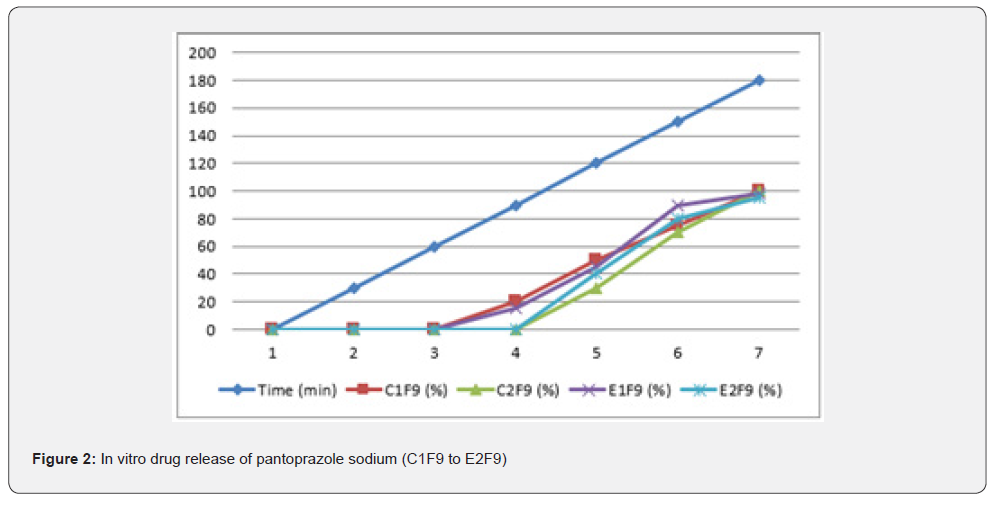
The first solution had a pH of 6.8, while the second solution had a pH of 1.2. The experiments into dissolving were carried out in a controlled setting with the use of a phosphate buffer and 1.2 N hydrochloric acid. The USP Type II rotating paddle dissolving device (Electro lab TDT-08L, India) was the apparatus that was used in this study. On the other hand, the C2F9 formulation produced the greatest results; the drug release started at two hours and reached a maximum of 99.72 when it was three hours. The remaining ones were as described below: release began in 3 hours and 96.42 minutes later, CIF3-90 minutes later, C2F3-2 hours later, 94.59 minutes later, E1F3-90 minutes later, E2F3-105 minutes later, 97.54 minutes later, C1F9-90 minutes later, 99.79 minutes later, EIF9-90 minutes later, 97.97 minutes later, E2F9-2 hours later, and 97.39 minutes later. The cumulative percentage releases of pantoprazole sodium tablets are shown in Table 7–14 and Figure 1&2 respectively.
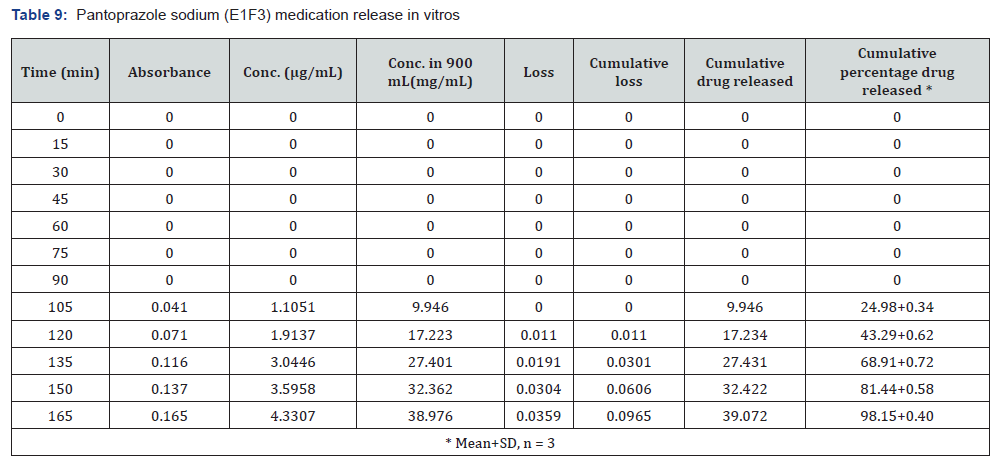
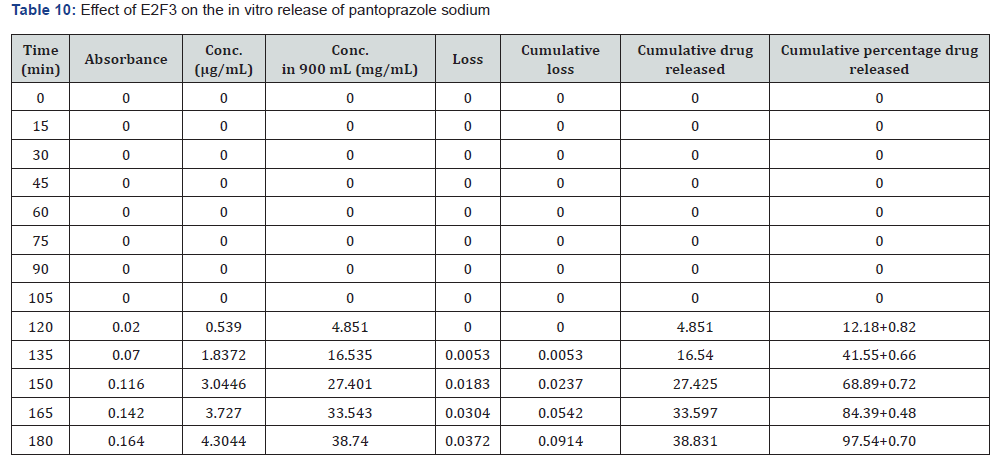
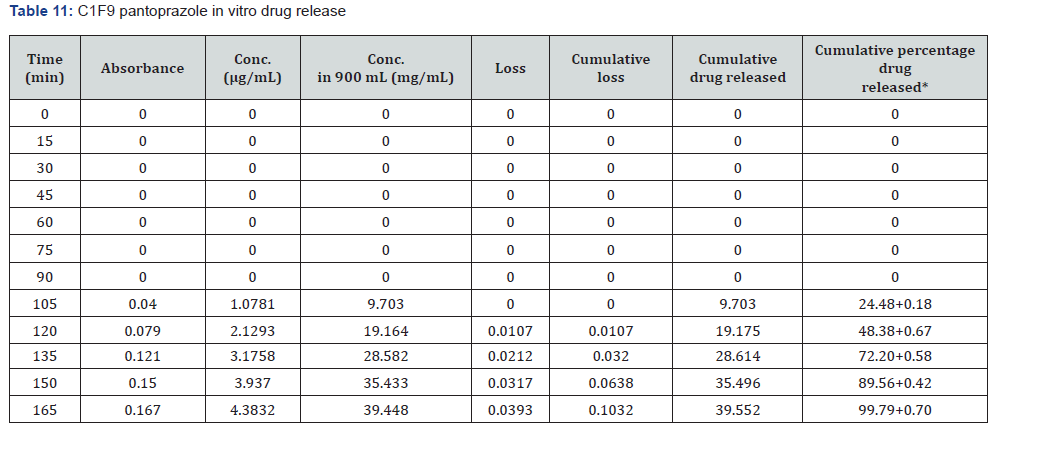
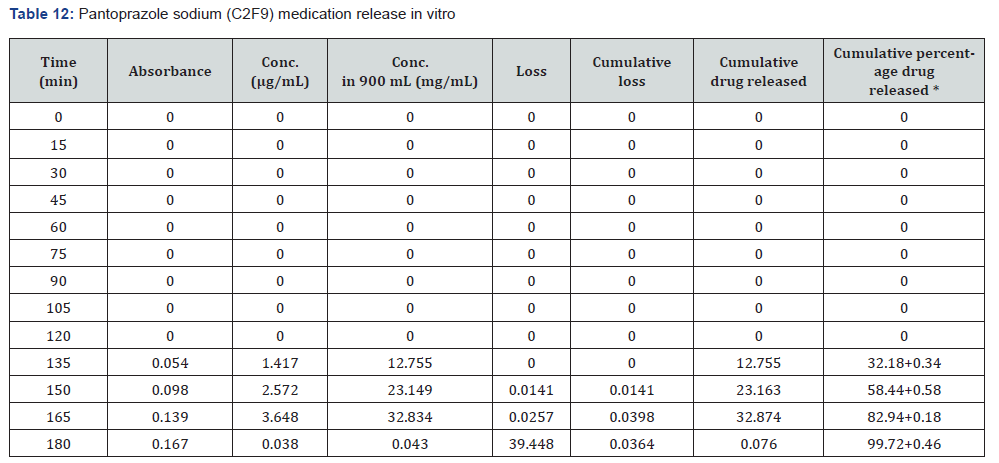
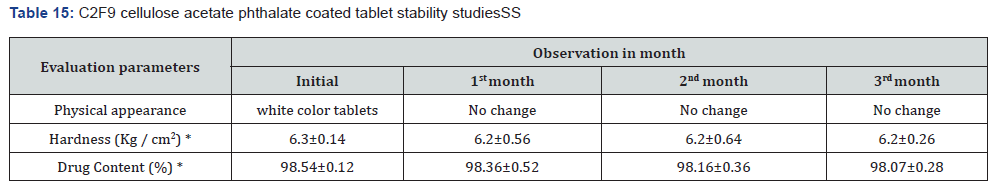
Stability studies: When establishing the expiry date of a formulation, one of the most important factors to consider is the stability of the dossage form under different environmental circumstances. Changes in the formulation’s physical appearance, colour, smell, taste, or texture are indicators that the medicine is unstable that may be seen. Following an examination of the release characteristics and physicochemical features of the coating films, Formulation C2F9 was selected for the purpose of conducting stability tests among the three enteric coated formulations. Based on the information provided in Table 15 the stability experiments were carried out at a temperature of 40±2 degrees Celsius and a relative humidity of 75±5 percent. All aspects of the tablets, including their overall appearance, their average weight, and their level of hardness, remained unchanged. The drug content of the samples that were evaluated after one, two, or three months of storage showed very little variation from the drug content that was present when the samples were first analysed. In light of the fact that the release profile did not change, it may be deduced that the formulation’s chemical and physical characteristics did not undergo any transformative changes. According to the data, it can be deduced that the tablets that were manufactured were stable and that they preserved their medical characteristics for a period of at least three months.
Conclusion
During the course of this study, pantoprazole sodium entericcoated tablets were successfully manufactured by using Cellulose Acetate Phthalate (CAP) and Eudragit L100 as coating polymers. Drug stability in gastric pH and release in intestinal pH was both accomplished by the use of these polymers, which successfully shielded the medications from the acidic environment of the stomach. Among all of the batches that were produced, the formulation C2F9, which was coated with 8% CAP, had the most favourable outcomes. It had the required dissolving profile, the suitable pre- and post-compression properties, and an excellent drug content within its composition. Pantoprazole’s acid-lability may be reduced by enteric coating, according to the findings, which would make the medication more stable and effective in the treatment of stomach ulcers and Gastroesophageal Reflux Disease (GERD).
References
- Shin JM, Sachs G (2008) Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep 10(6): 528-534.
- Sachs G, Shin JM, Howden CW (2006) The clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther 23(2): 2-8.
- Kromer W (1995) Clinical pharmacology of pantoprazole. Alimentary Pharmacology & Therapeutics 9(Suppl 2): 3-10.
- Cole G, Hogan J, Aulton M (2002) Pharmaceutical coating technology. Taylor & Francis.
- Rowe RC, Sheskey PJ, Quinn ME (2009) Handbook of pharmaceutical excipients (6th ed). Pharmaceutical Press.
- Aulton ME, Taylor KMG (2017) Aulton’s pharmaceutics: The design and manufacture of medicines (5th). Elsevier.
- Patel T, Singh A, Solanki S, Kapasiya S, Patel R, et al. (2024) Formulation and evaluation of enteric coated tablets of pantoprazole. IJPPR 9(2): 45-53.
- Gobinath T, Kamalakkannan V, Sambathkumar R (2014) Formulation and evaluation of enteric coated tablets of pantoprazole. Jchps 7(3): 176-184.
- (2022) United States Pharmacopeia (USP). USP 46-NF 41. United States Pharmacopeial Convention.
- (2019) ICH Q1A(R2) Stability testing of new drug substances and products. International Council for Harmonisation (ICH).
- Sumit C, Sibaji S, Sujit D (2009) Formulation Development and Evaluation of Pantoprazole Enteric Coated Tablets. Int J Chem Tech Res 1(3): 663-666.
- Anroop N, Rachna G, Rachna K, Shery J, Mahesh A (2010) Formulation and Evaluation of Enteric Coated Tablets of Proton Pump Inhibitor. J Basic Clin Pharm 1(4): 215-221.
- Neelam DK, Prafulla SC, Rajesh J (2011) Innovations In Tablet Coating Technology: A Review. IJABPT 2(1): 214-217.
- Senthil K, Ashokkumar S, Ezhilmuthu RP (2010) Formulation and evaluation of didanosine enteric coated sustained release tablet. J Biomed Sci and Res 2(3): 126-131.
- Rupesh K, Archana D, Kajale, Keshao P, Giradkar V (2010) Formulation and development of enteric coated dosage form using ketorolac tromethamine. IJPRD 2(8): 126-135.
- Martin A (2001) Micromeretics. In: Martin A, ed. Physical Pharmacy, Baltimores, MD: Lippincott Williams and Wilkins pp. 423-454.
- Liberman H, Lachman L (1991) The Theory and Practice of Industrial Pharmacy. 3rd Bombay: Verghese Publication House pp. 171-193.
- Saffar M, Rajeev S, Sophia D (2007) Comparative in vitro evaluation of commercially available pantoprazole tablets. Kathmandu University Journal of Science, Engineering and Technology 1(3): 1-7.
- Bozdag S, Çalis S, Sumnu M (1999) Formulation and stability evaluation of enteric-coated omeprazole formulations. STP. Pharma Sciences 9 (4): 321-327.
- Singh C, Kumar R, Agarwal K, Nema K (2009) Development and evaluation of enteric coated tablet containing diclofenac sodium. IJPSN 2(1): 443-449.






























