Abstract
It is now necessary to target the cells involved in the onset and progression of diseases specifically due to developments in molecular pharmacology and our growing understanding of the mechanisms underlying the majority of diseases. This is particularly true for the majority of terminal illnesses that call for therapeutic drugs with a host of negative side effects, necessitating precise tissue targeting to reduce internal exposure. Current drug delivery systems (DDS) are designed using cutting-edge technology to minimize off-target accumulation in the body and maximize therapeutic efficacy by accelerating systemic drug delivery to the precise target region. They so have a crucial role to perform when treating and managing illness. Advanced drug delivery systems (DDS) are more advantageous than traditional drug administration systems because of their improved efficacy, automation, performance, and precision. They are composed of multifunctional nanomaterials or tiny devices with long circulating half-life that are biocompatible, biodegradable, and have high viscoelasticity. As a result, this review offers a thorough understanding of the development of drug delivery systems’ technological history. It provides an update on the newest drug delivery systems, their therapeutic uses, the challenges in using them, and the directions that will hopefully lead to better performance and utilization in the future.
Keywords: Drug; Drug delivery system; Nanomaterials
Abbreviations:DDS: drug Delivery Systems; EPR: Enhanced Permeability and Retention; PDDS: Polymer-Based Drug Delivery Systems; PEG: Polyethylene Glycol; SEDDS: Self-Emulsifying Drug Delivery Systems; SNEDDS: Self-Nanoemulsifying Drug-Delivery Systems; CoS: Co-Surfactants; RBC: Red Blood Cells; PAA: Polyacrylic Acid; TPP: Tripolyphosphate Salts; CSNP: Chitosan Nanoparticles; FHB: Fibroblasts; SLN: Solid Lipid Nanoparticles; FDOF: Fast Dissolving Oral Films; FDT: Fast-Dissolving Tablet; ODDS: Osmotic Drug Delivery Systems; VDDS: Vesicular Drug Delivery Systems; AM: Asenapine Maeleate
Introduction
Saturated Technology-based drug delivery systems prepare and store drug molecules into forms that are appropriate for administration, such as tablets or liquids. They enhance the delivery of medications to the precise targeted place within the body, optimizing therapeutic efficacy and reducing the likelihood of off-target accumulation within the body. There are several ways that drugs can enter the body; they include, but aren’t limited to, the oral, buccal, and sublingual routes; nasal and ophthalmic; transdermal and subcutaneous; anal and transvaginal; and intravesical. The drug’s constituents are what give it its physiochemical characteristics and cause the alterations in the physiological systems that it affects when consumed Patel R et al. [1].
Due to improved systemic circulation and control over the drug’s pharmacological action, DDS has been used successfully in the last few decades to treat illnesses and promote health. Controlled release is a concept that emerged from the development of pharmacology and pharmacokinetics, which demonstrated the significance of drug release in influencing therapeutic success. Since its initial approval in the 1950s, the controlled-release formulation of a medication has generated a lot of interest because of its notable benefits over conventional medication. It distributes medication for a given amount of time at a predetermined rate. Furthermore, controlled drug delivery systems have a daysto- years duration since they are not impacted by physiological circumstances. With constant or variable release rates, it also offers spatial control over drug delivery. In addition, it decreases medication toxicity and enhances drug solubility, target site accumulation, efficacy, pharmacological activity, pharmacokinetic characteristics, patient acceptance, and compliance Liechty WB et al. [2].
In order to provide more easy, regulated, and targeted distribution, a number of drug delivery systems (NDDS) have recently been developed using advanced technology. The unique characteristics of every medication delivery system dictate its release mechanism and rate. This is mostly because of the variations in their morphological, chemical, and physical properties, which ultimately influence how well-suited they are for different types of drugs. Diffusion, chemical reaction, solvent reaction, and stimulus control have been found to be the main release mechanisms in these studies. For example, the medicine can readily pass via the lymphatic system and porous blood vessels to reach the target tissues, since most cancer cells can multiply through these openings. The term “Enhanced Permeability and Retention” (EPR) describes this. Patel SM et al. [3].
Drug Delivery Carriers
vehicles for the delivery of drugs: Colloidal drug carrier systems, such as micelle solutions, vesicles, liquid crystal dispersions, and nanoparticle dispersions made up of tiny particles with a diameter of (10–400) nm, have demonstrated great potential as drug delivery techniques. Developing materials with desired properties, such as extended shelf life, low toxicity, and ideal drug loading and release qualities, is the aim of this type of formulation development. Drugs that are incorporated into a system participate in its microstructure and have the ability to influence it through molecular interactions, especially if they exhibit meso-genic or amphiphilic properties. Amphiphilic block copolymers are suitable for use in drug delivery applications because of their versatility in terms of chemical composition, total molecular weight, and block length ratios. This allows for modifications of the size and form of the micelles. Micelles in block copolymers with cross-linkable groups added can be stabilized, and their temporal control can be improved. The replacement of block copolymer micelles with specific ligands is a very promising strategy to a greater range of places of action with significantly higher selectivity (Adepu S et al. 2021).
Drug Delivery System
The process of administering a pharmaceutical substance to humans and other living things in order to have a therapeutic effect is known as drug delivery. A drug delivery system is characterized as a formulation or a device that regulates the rate, time, and site of drug release in the body to facilitate the entry of a therapeutically active chemical and enhance its safety and efficacy. Poor bioavailability, solubility, in vivo stability, intestinal absorption, prolonged and targeted delivery to site of action, therapeutic effectiveness, side effects, and plasma fluctuations of drugs that either fall below the minimum effective concentrations or exceed the safe therapeutic level are some of the issues that most drug delivery systems face. (Tiwari et al. 2012 and Gokhan et al. 2011). Most common of drug delivery includes oral (through mouth), topical(Skin), Trans-mucosal (vaginal, nasal, buccal, sublingual, ocular, rectal), inhalation routes and parenteral (Injection into systemic circulation).
The drug delivery system can further be divided into 2 main
types:
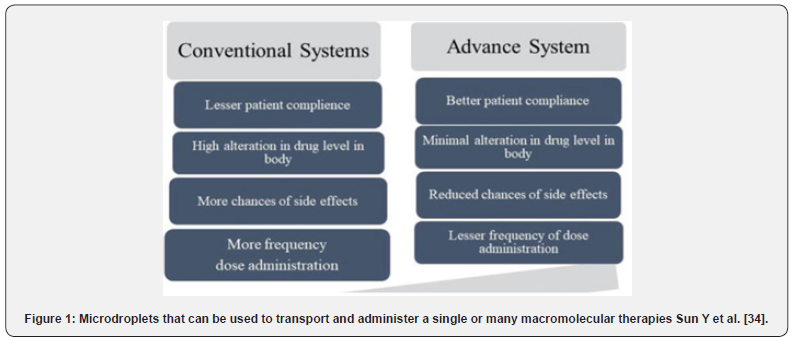
a) Conventional drug delivery system.
b) Advanced drug delivery system (Figure 1).
Evolution of drug delivery
People relied on medicinal herbs in the past. They were helpful, but their medication administration lacked uniformity, homogeneity, and specificity. All medications were created and kept in pill or capsule forms prior to the use of controlled drug administration. When it comes into touch with the fluids of digestion, it dissolves, penetrates the gut wall, and is subsequently taken up by those in the bloodstream and absorbed into the circulation. The kinetics of the drug release could not be controlled. Rhazes and Avicenna introduced coated technology in an effort to mask the bitter taste of medications. The drug’s own release rate was changed by this coating technique. However, it was implemented in the 10th century as pearl-coated, silver, and gold-coated tablets. Advanced coating techniques including keratin, shellac, sugar, enteric coating, and pearl coating were also introduced in the 20th century; however, keratin and shellac proved useless because of their instability storage and high pH, which prevented them from dissolving sufficiently in the small intestine. A novel enteric-coating material has been developed that contains polymeric cellulose acetate phthalate and dissolves at an extremely weakly alkaline pH, similar to the small intestine. This makes it ideal for use in enteric controlled release applications (Malm et al. 2017).
The first generation was quite productive; its main goals were the creation of controlled drug release mechanisms and a wide range of oral and transdermal controlled-release formulations for clinical application. When Lipowski placed the medication and coat on pills made of enteric polymers, which resemble beads, he first presented a patent oral sustained-release formulation in 1951.As an alternative, the medicine will release slowly on a regular and periodic basis. The oral predetermined-release formulation known as “Spansule technology,” which progressively maintains and regulates a drug’s kinetic release, was further developed in 1952. This formulation is made up of hundreds of tiny pellets that are filled with drugs and have different amounts of naturally occurring water-soluble wax on each pellet. The drugloaded beads are released upon consumption when the exterior capsule quickly dissolves and the waxy coating around the beads progressively dissolves as they pass through the GI system. This became very popular since it reduced the dose schedule and increased patient compliance and convenience. By substituting more consistent synthetic polymers for the wax, this method was advanced (Smith et al. 1952).
The first type of nanotechnology was identified as liposomes, or lipid vesicles, in the 1960s.
The second generation of drug delivery formulations was quite successful; however, the anticipated therapeutic outcomes were not achieved. Constant drug release rates, self-regulating, long-term depot formulations, and formulations based on nanotechnology-particularly nanoparticle formulations-were among the drug delivery technologies that the researchers were interested in creating. Drug formulations for peptide/protein combinations with long-term depot-sustained release were created in this era. Smart polymers and hydrogels have also been designed to stabilize drug delivery systems that are impacted by physiological variables including glucose, pH, temperature, and electric field. Additionally, attempts were undertaken to create targeted nanotechnology DDS for cancers and gene delivery by employing biodegradable polymers in nanoparticle shapes like lipids, dendrimers, polymeric micelles, and chitosan.
The controlled release technology of today represents the third generation of medication delivery systems. It must get past the physicochemical and biological difficulties that accompanied the preceding drug delivery technologies in order to be advantageous and successful. While the biological barrier challenges are related to systemic drug distribution issues, the physiochemical challenges are generated by therapeutic proteins and peptides’ high molecular weight, low water solubility, and difficulty achieving targeted and controlled drug release (Figure 2 & 3).
Current medication delivery systems and applications
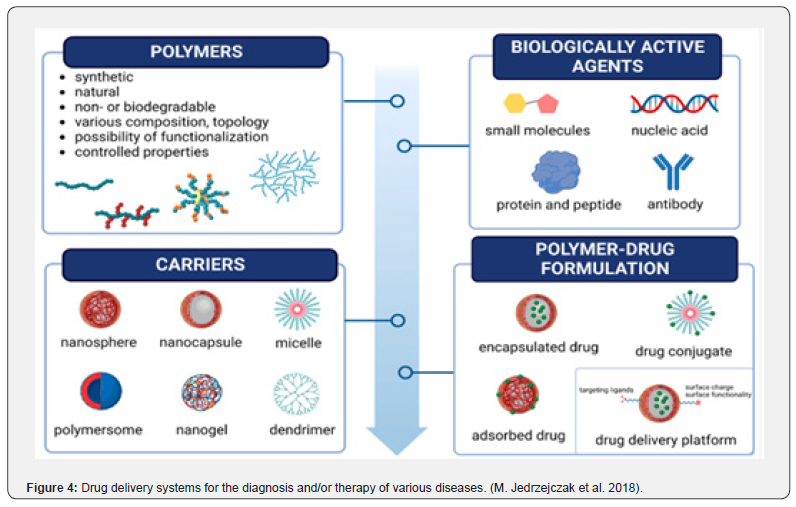
Recent years have seen a significant advancement in the effective creation of drug delivery systems, particularly in chemotherapy, based on the use of organic, inorganic, and hybrid nanoparticles as drug carriers for active targeting. Newer drug delivery systems (DDS) are designed with improved characteristics such stability, toxicity, prolonged delivery, efficacy, enhanced permeability, solubility, and particular site targeting. In comparison to traditional dosage forms, they can greatly enhance the therapeutic agent’s performance. It is acknowledged that the most recent advancements in drug delivery system development are those related to contemporary drug delivery systems (Figure 4).
The application of DDSs has enhanced the clinical efficacy of numerous medications and facilitated the development of novel treatments, including siRNA and anti-cancer treatments. Nonetheless, drug delivery systems still need to solve a few issues before they may be used in clinical settings. The delivery of complex therapeutic molecules is hindered by biological barriers, which will need to be overcome by the next generation of DDSs. In the meantime, less invasive systems that secrete biomolecules into particular tissues at specific times and concentrations-and sometimes for an extended period of time-will need to be used. Polymer-based drug delivery systems (PDDSs) will continue to be the most active area of biomedical research in university and pharmaceutical laboratories, and advancements in this sector will be the key to breaking through current barriers (Adepu S et al. 2021).
Polymer-Lipid Hybrid Nanoparticles Drug Delivery System
Nanocarriers are becoming more and more popular as drug delivery systems due to their enhanced storage stability, better ability to target disease cells, longer drug release, and better encapsulation capacity. Polymeric and liposome nanoparticles are the most extensively used of the widely acknowledged nanoparticles currently being employed for medication delivery. The polymeric nanoparticle, a polymer-based nanoparticle, was able to overcome this limitation by demonstrating high encapsulation/drug loading ability as well as stability. Liposomes, lipid-based nanoparticles, showed excellent biocompatibility but still experienced drug leakage and instability upon storage Ulbrich K et al. [4] (Figure 5).
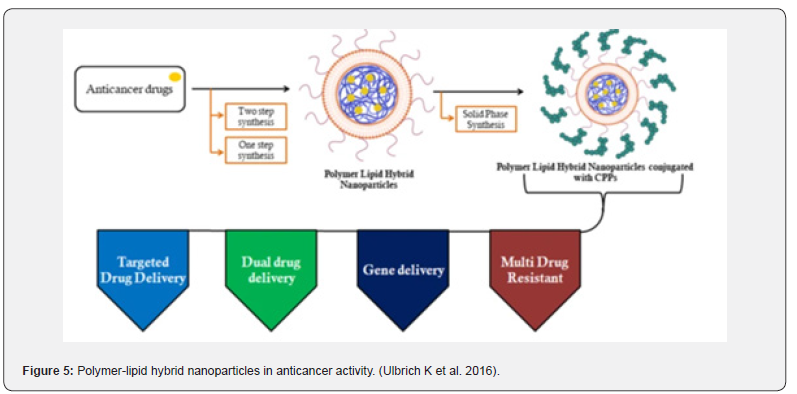
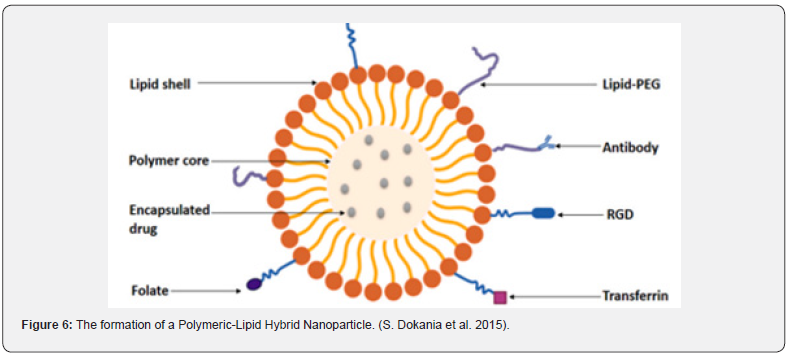
But it also had a lesser biocompatibility, which was one of its shortcomings. The term “Polymer-lipid hybrid nanoparticles” refers to the hybrid system that researchers have created to combine the special qualities of the two classes of nanoparticles in order to overcome these drawbacks and produce an effective nanomaterial. The needs of high encapsulation, miniaturization, little drug leakage, sustained drug release, high storage stability, and small particle size were all met by this hybrid system. This technology is currently being used for several therapeutic objectives in addition to diagnostic applications due to its effectiveness. is composed of three separate parts, which are as follows: A polymeric core capable of efficiently encapsulating both hydrophilic and hydrophobic medications. A lipid-polyethylene glycol (PEG) that is found in the outer part and covered by a lipid layer to provide increased steric stability, prevent immune recognition, and increase time for circulation, as well as a lipid shell that provides biocompatibility and high stability, all contribute to this high sustained release which is shown in Figure 3 Vugmeyster Yet al. [5].
Self-micro emulsifying drug-delivery system
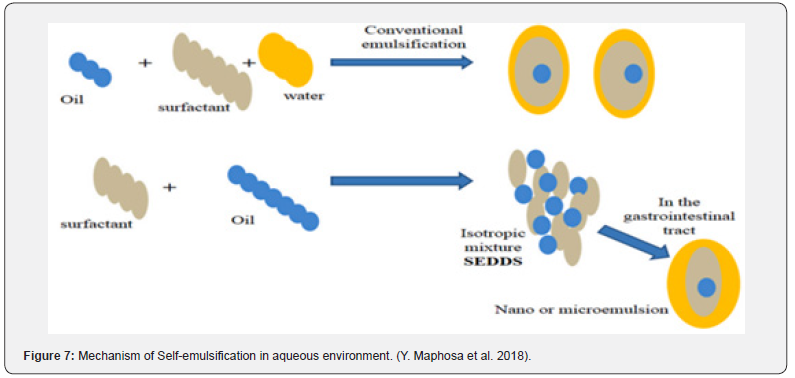
Lipid-based carriers are available in multiple forms, such as microemulsions, suspensions, dry emulsions, and selfemulsifying drug delivery systems (SEDDS). It has previously been observed that SEDDS can integrate hydrophobic medicines. Self-micro emulsifying drug-delivery systems (SMEDDS) and selfnanoemulsifying drug-delivery systems (SNEDDS) are the new versions of SEDDS. Conversely, emulsions are produced by mixing a liquid phase with macroscopic particles with another liquid phase made of surfactant. Additionally, these solutions are semitransparent (sometimes hazy), thermodynamically unstable, and have characteristics similar to viscous liquids (Figure 6).
Emulsions are of three types, viz: water-in-oil, oil-in-water, and multiple emulsions. Additionally, Conventional micro- or nano emulsions further differ from SMEDDS in that they selfemulsify after oral consumption. In microemulsions, two types of emulsifying agents are used: surfactants (S) and co-surfactants (CoSs) (Figure 4). On the other hand, CoS is largely soluble in the oil phase, whereas surfactant is mostly soluble in water. However, the creation of nano emulsions with droplet sizes less than 100 nm calls for the use of chemical or mechanical energy. Despite being categorized as kinetically stable because of their incredibly low rate of destabilization, nano emulsions stand out for their longlasting stability (measured in months). As a result, it has been demonstrated that nano emulsion globules remain stable across a range of conditions, such as different dilutions and temperatures, but microemulsions are primarily affected by dilutions and temperature Glassman PM et al. [6] (Figure 7) (Table 1).
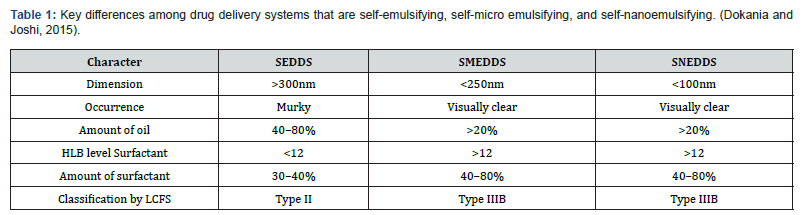
HLB: Hydrophile-lipophile balance, LCFS: Lipid classification formulation system, SEDDS: Self-emulsifying system, SMEDDS: Self-microemulsifying system, SNEDDS: Self-nano-emulsifying system.
Red blood cell membrane-camouflaged nanoparticles drug delivery system
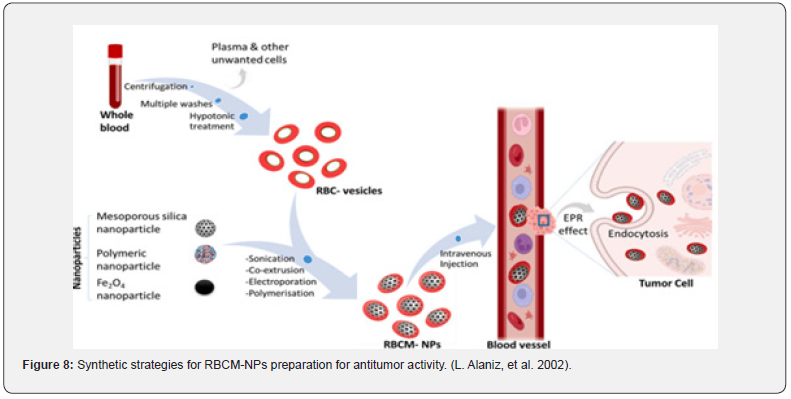
Over time, researchers have come to understand the potential advantages of nanotechnology in significantly enhancing pharmaceutical delivery techniques. Nanoparticles disguised like red blood cell membranes are a novel class of drug delivery vehicles. Red blood cells (RBCs) can be used as an effective method as a nanoparticle camouflaging material due to their nature and biological significance. Red blood cells are one of the largest circulating cells in the body, and because of their extended circulating half-life, biodegradability, and lack of immunogenicity, so they are the perfect medium for delivering drugs. Research has shown that engineered red blood cells (RBCs) are great vehicle for a wide range of bioactive substances, such as proteins, macromolecules, drugs, and enzymes. Red blood cell membranes are readily available thus they act as a “camouflage,” enabling nanoparticles to combine the advantages of the nanomaterial with the native membranes’ benefits. Numerous methods have been devised to encapsulate medicinal substances onto red blood cells (RBCs) while maintaining their physiological and structural integrity. After being injected, the coated nanoparticles will behave like RBCs and interact with the surroundings to create long-lasting systemic circulation. The most popular technique for producing nanoparticles with RBC camouflage is sonication. Additional techniques for fusing red blood cells with nanoparticles consist of extrusion, microfluidic electroporation, and in-situ polymerization. Before fusion, the RBC membrane-derived vesicle is extracted from fresh whole blood of an organism by hypotonic therapy (dialysis, hemolysis, or dilutions). The hypotonic therapy aids in the elimination of unwanted cells and plasma (T. Hanley et al.,2021) (Figure 8).
In-situ gel drug delivery system
One of the most cutting-edge drug delivery techniques is
the administration of medications via in-situ gel. The in-situ gel
drug delivery system helps with the controlled and sustained
release of drugs, as well as improved patient compliance. Before
entering the body, formulations in solution form often undergo
a transformation into gel form under specific physiological
conditions. A solution can become gelled through a combination
of stimuli, including pH alteration, temperature modulation, and
solvent exchange. Numerous methods of research have been
employed, including parenteral, nasal, injectable, vaginal, rectal
ocular, intraperitoneal, and oral. Polymers, both synthetic and
natural, are used to make in situ gel drug delivery devices. Four
processes have been identified as responsible for the formation of
in-situ gel biomaterials:
a. changes in temperature and pH
b. changes in the biomaterials’ physical properties, such as
swelling and solvent exchange.
c. biochemical modification, including enzymatic and
chemical reactions; and
d. photo-polymerization. (R. Bashir et al.,2021).
Applications of in-situ gel delivery system
Oral in-situ gel delivery systems: This strategy focuses on the administration of medication at specific sites in the gastrointestinal tract by using pH-sensitive hydrogels. Silicone microsphere hydrogels that release prednisolone into the stomach medium or have gastroprotective qualities have been created using varying concentrations of crosslinked polyethylene glycol (PEG) and derivatives of polyacrylic acid (PAA). Natural polymers like xyloglucan, pectin, and gellan gum are used in the oral in situ gel dispersion processes. The formulation based on pectin was developed to ensure the continuous distribution of Metforminloaded pectin (PCM). An organic solvent is not required because pectin is soluble in water.
Injectable in-situ gelling system: This in-situ forming gel has been utilized to minimize pelvic discomfort, bowel obstruction, and infertility in order to prevent postoperative peritoneal adhesion. Creating dosage forms such as injectable or implanted delivery systems is the only apparent method of administering medications for extended release. Typically composed of poloxamers, thermo-reversible gels are the most often used type. The production of regulated medication delivery for systemic absorption may benefit from these dosage forms. Further applications for poloxamer gels include the delivery of human growth hormone intramuscularly and subcutaneously, as well as the creation of an injectable single-dose lidocaine with a prolonged half-life. Poly (D, L-lactide)/1-methyl-2-acrylic acid blends are used in Pluronics’ newly created range of depot protein injectables with controlled release formulation compounds of pyrrolidone (S. Dash et al. 2021).
Nasal in-situ gelling systems: Polymers such as gum glean, and gum xanthan are used in nasal in-situ manufacturing. Research has been done on the effectiveness of mometasone furoate as an in-situ gel in the treatment of allergic rhinitis. A model of allergic rhinitis utilizing sensitized rats was used to demonstrate the in vivo effects of in-situ gel on antigen-induced nasal symptoms A. Paul et al. [7].
Ophthalmic in-situ gelling systems: Within the ocular delivery system, natural polymers such as xyloglucan, alginic acid, and gellanic gum are used. The local ophthalmic administration technique uses a number of combinations of anti-inflammatory, antimicrobial, and autonomic medications to minimize the stress of intraocular glaucoma. The quick turnover and dynamics of tear fluid have led to its development as a solution to the bioavailability issue with ocular insitu gel. In ocular preparations, viscosity enhancers such as Carboxymethyl Cellulose, Polyvinyl alcohol, Carbomers, and Hydroxypropyl methyl cellulose are used to improve the viscosity of the drug formulations, resulting in higher bioavailability and precorneal residence time. Enhancers of chelating agents’ penetration are used to encourage the infiltration of ocular materials like preservers and surfactants (R. Sheshala et al.,2015).
Rectal in-situ gelling systems: Many medications that are packaged as liquids, semisolids (liniments, emulsions, and froths), or suppositories in solid administration formulations can be prescribed using this technique. Traditional suppositories could cause discomfort during penetration. Additionally, suppositories can migrate higher into the gut due to their inability to be adequately maintained at a single rectum site, which enables the drug’s first-pass action to be observed M. Bialik et al. [8].
Combined Drug Delivery Approach
Due to its increased complementarity and wider target specificity, combination therapy is now more practical for improving therapeutic efficacy and improving clinical outcomes. In order to combat multidrug resistance, combined medication delivery has become a popular strategy in cancer research and treatment. According to reports. The combined drug administration technique decreases drug dosage and adverse effects while increasing drug efficiency and decrease in drug resistance are maintained. A study on combination therapy for magnetic hyperthermia therapy was carried out, and the results were positive. The chitosan nanoparticles (CSNPs) were crosslinked ionically using tripolyphosphate salts (TPP). Three distinct ferrofluid concentrations were encapsulated along with a consistent concentration of 5-fluorouracil (5-FU) to create the magnetic CSNPs. They employed human glioblastoma A-172 cells, which are cancer cells, and normal cells called fibroblasts (FHB) H. Wang et al. [9].
Micro electromechanical systems (MEMS) for drug delivery
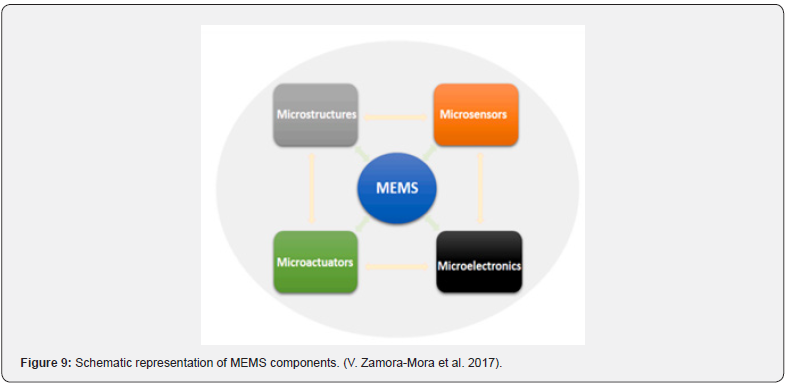
MEMS technology is widely used in areas like inkjet printing, motion detection, accelerometers, actuators, and medication delivery. By using microfabrication processes, the devices made possible by this technology can create mechanical, electromechanical, and micro- or nano-sized implants. It’s interesting to note that by giving designers significant control over the topography, microarchitecture, and size of the final devices, these strategies improve the effectiveness of these devices. The most often utilized materials and design techniques for these MEMS-based devices are numerous. combine inventive combinations of different micromachining processes, including lithography (a patterning process), etching (a subtractive process), deposition (an addictive process), ink jetting, ion implantation, oxidation, and micro molding. With the use of MEMS technology, miniaturized systems made of silicon, glass, metals, and nitrides can be created, in addition to polymers, micropumps, sensors, microvalves, reservoirs, actuators, and high-performance CPUs. Together, these several parts work in concert to provide MEMS devices their widely acknowledged multifunctionality and precision in comparison to other traditional drug delivery methods. Actuators, for example, are primarily responsible for the drug release process by applying pressure to the drug reservoir, hence facilitating drug release. Each of these aspects’ functions strategically. With a single reservoir architecture, one medicine can be contained in a comparatively big port. Due to its refillable nature, it can hold a comparatively greater volume of medication and is ideal for extended use. Different medications can be included into multi-reservoirs since they consist of various ports that store drugs independently inside the same substrate. They would need to undergo repeated replacements because there are no refilling options, which makes them less appropriate for longterm use. Additionally, microvalves are used to switch on and off the delivery device, seal, and regulate the rate of fluid flow ( V. Zamora-Mora et al.,2017).
Because their multifunctional components are integrated into their smaller sizes, MEMS-produced drug delivery devices have various advantages over traditional distribution techniques, including improved performance, automation, precision, and efficacy. It also contributes to less painful and invasive attributes of the devices. Furthermore, MEMS-based devices enable the automatic release of numerous medications from reservoirs, as well as the maintenance of drug stability during encapsulation and customizable, continuous distribution. Moreover, it improves the medication’s bioavailability, localized release, sustainability over an extended length of time for drugs needing complex dosage, individualized dosing profiles, and capacity to operate under sustained zero-order kinetics. The integration of wireless electronics to remotely control and track the device’s operation as well as the patient’s response has been predicted to increase device security risks, medical packaging, and regulatory complexities, so MEMS-drug delivery devices may present technical challenges F. Silva et al. [10] (Figure 9) reported that nanoparticles loaded in cells were more effective than the nanoparticle drug delivery system on a specific combination drug delivery technique that focused on utilizing cells in combination with nanoparticles. Improved therapeutic efficacy, prolonged half-life, sustained drug release, and reduced immunogenicity and cytotoxicity were all demonstrated by the cell-based therapy. Its migratory and chemotaxic potential were unaffected by the combination of nanoparticles and exploit cells. Because of this, the combined medication delivery approach is thought to be a viable strategy for medical therapy and pharmacological research.
Targeted Drug Delivery System
Because of the link between saturated fatty acids and elevated serum low-density lipoproteins, it was postulated that the dietary intake of saturated fatty acids contributes to cardiovascular disease.69 However, a comparison of two populations with similar intakes of saturated fatty acids and cholesterol-France and Finland-revealed different cardiovascular mortality rates. This study highlights that certain saturated fatty acids (stearic acid and medium-chained saturated fatty acids less than 12 carbons), and balanced consumption with monounsaturated fatty acids, play an important role in cardiovascular health K Rani et al. [11]. Literature focusing on the quality of saturated fatty acids links the intake of stearic acid and medium-chained fatty acids (less than 12 carbons) to increases in larger, more buoyant LDL particles, which present a lower risk for cardiovascular disease compared with smaller, dense LDL particles, despite cholesterol content.72–74 Therefore common cardiovascular risk factors, such as dyslipidemia, obesity, and metabolic syndrome, are not perpetuated by certain SFAs. Specifically, stearic acid (octadecanoic acid C18:0) and medium-chain saturated fatty acids decrease intestinal cholesterol absorption. (J. Dupont et al., 2003).
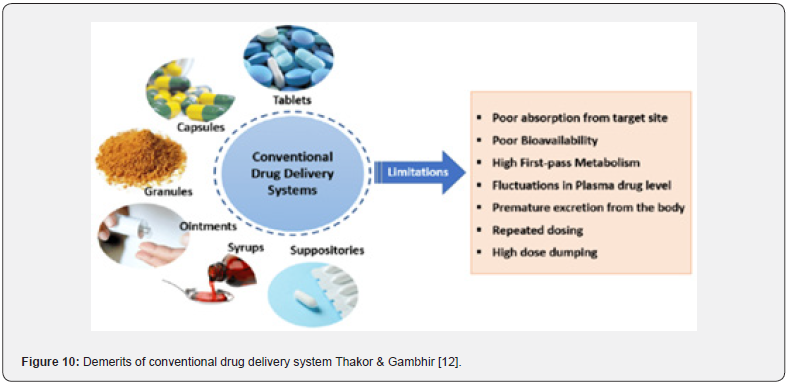
This method is a sophisticated technique that has been used recently because of its decreased negative effects and efficiency. This method is designed to distribute medications in a specific order, which increases the concentration of the medicine as it goes to the target location. The drug’s strength and efficacy remain unchanged even when the dosage is decreased to reduce negative effects. Among other drug carriers used in this method are liposomes, artificial cells, neutrophils, biodegradable microsphere polymers, soluble polymers, and micelles. Due to its effectiveness, particularly in fighting against cancer, this treatment is becoming more and more widely accepted. (Murugan et al.,2016) (Figure 10) (Table 2).

Nanoparticulate Drug Delivery Systems
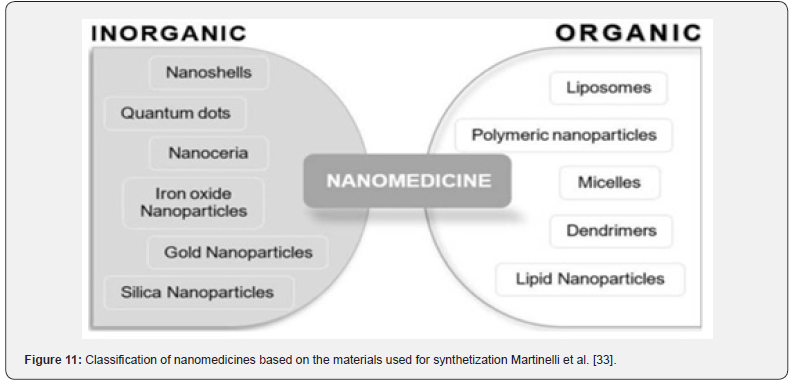
Typically, nanoparticulate drug delivery systems consist of two primary components: the therapeutic substance being delivered and the nanoparticle itself. To prevent devaluation and denaturing, the medication is either covalently bonded to the surface or, alternatively, is encapsulated and contained by the nanoparticle. Thakor & Gambhir [12]. The ideal particle size is around 100 nm tiny, which allows for immediate lymphatic system clearance, blood brain barrier penetration, and sufficient drug delivery because of a large surface area. In order to extend blood circulation, water-soluble polymers like polyethylene glycol (PEG) or polysorbate 80 have been used in polymer coating more recently. Typically, nanoparticulate drug delivery systems consist of two primary components: the therapeutic substance being delivered and the nanoparticle itself. To prevent devaluation and denaturing, the medication is either covalently bonded to the surface or, alternatively, is encapsulated and contained by the nanoparticle. Thakor & Gambhir [12]; Rizvi and Saleh, 2018) (Figure 11) (Table 3).
Types of nanoscale drug delivery systems
Polymer-based nanoparticles: Although the word “polymeric nanoparticle” can refer to a variety of polymer nanoparticle forms, the major descriptions of nanospheres and nano capsules. Nanospheres are essentially solid, spherical particles that range in size from 10 to 200 nm. They are composed of a homogenous structure and a matrix system. By contrast, nano capsules are vesicular systems with a polymer membrane or coating encircling an aqueous or somewhat oily liquid core. The medicine is enclosed in the inner core in either dissolved or dispersed form, trapped in the polymeric membrane, and spread throughout the pseudo-phase. The majority of the wallforming polymer is composed of a biodegradable substance that can be synthetic or natural. Polymers such as gelatin, chitosan, polylactide, and polylactide-co-glycolic acid are often utilized. Although the most often used method for creating nano capsules is nanoprecipitation, six other techniques have been documented: polymer coating, solvent displacement, emulsion coacervation, double emulsification, and emulsion diffusion Frank et al. [13]; and Lai et al., 2014).

The entrapment of the API can result in a number of additional benefits, as nano capsules as drug vehicles have been studied in several studies for a variety of routes of administration, demonstrating their diversity. The polymer in the nano capsule interface, on the other hand, provides both chemical stability and photoprotection. Moreover, since the therapeutic substance is typically absorbed while imprisoned within the nano capsules, there is an improved interaction with tissues and cells. The bioavailability and efficacy of drugs are increased, and side effects are decreased when nano capsules are used as a delivery mechanism. Few products are on the market at the moment, despite the fact that a lot of research has been published Frank et al. [13] and Yurgel et al., 2013).
Solid lipid-based nanoparticles: The goal of creating solid lipid-based nanoparticles was to replace polymeric nanoparticles, liposomes, and emulsions as a drug delivery method. As shown in Figure 12, there are actually two main types of solid particle matrices that differ in composition: solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) (Figure 12). Classification of solid lipid nanoparticles and nanostructured lipid carriers based on the distinct character of the matrix. This table was modified from Ganesan and Narayanasamya (2017) (Ganesan and Narayanasamy, 2017).
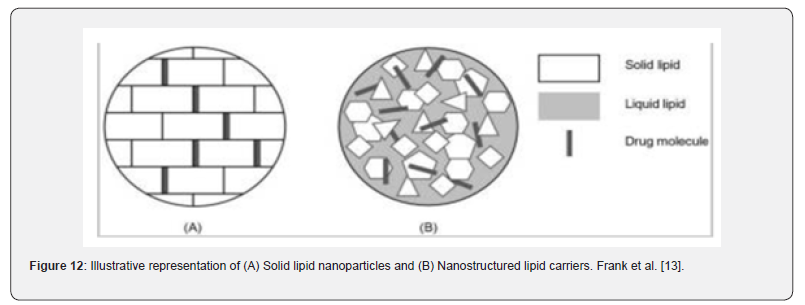
Solid lipid nanoparticles:
Type 1: Nature of Matrix-Homogenous matrix model
Type 2: Nature of Matrix- Drug enriched shell model
Type 3: Nature of Matrix- Drug enriched core model.
Nanostructured lipid carriers:
Types:
I. Imperfect: Nature of Matrix- Imperfectly structured
solid matrix.
I. Amorphous: Nature of Matrix- Structureless solid
amorphous matrix.
III. Multiple: Nature of Matrix- Multiple oils in fat in water.
Gels
Hydrogels are formed of cross-linked, hydrophilic polymers from natural or synthetic sources that imbibe enormous volumes of water, forming a three-dimensional network with porous properties and high levels of elasticity. Since their swollen state resembles living tissue, they have high biocompatibility and can be used in a wide range of applications. The term “stimuliresponsive” or “smart” describes hydrogels that have the ability to change their volume in response to environmental changes. For example, a self-regulated release of insulin is made possible by the use of glucose sensors like lectin to control swelling, or deswelling Lee et al. [14]. As shown in Figure 13, polymers derived from natural or synthetic sources can be cross-linked chemically or physically.
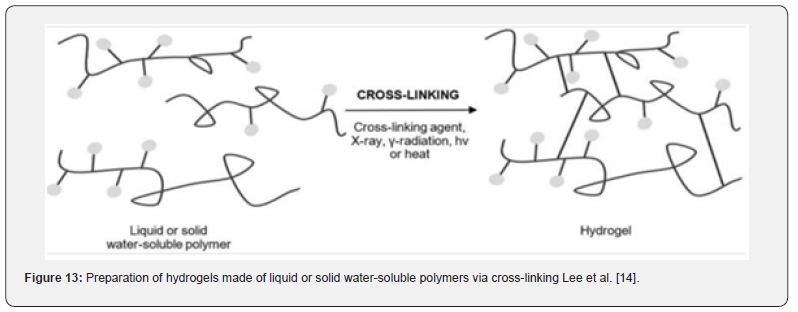
Since harmful cross-linking chemicals are frequently not needed for manufacture, physical hydrogel has attracted more attention recently. This process is typically reversible due to conformational changes and is accomplished by processes such chain aggregation, crystallization, hydrophobic association, and hydrogen bonding, among others. (Ullah et al., 2015; Caló and Khutoryanskiy, 2015). With the exception of their nanoscale size, anogels-also referred to as the hydrogels of the future erahave properties and a structure similar to hydrogels. Either the kind of three-dimensional network cross-linking or the response to a specific stimulus determines the classification. The pH or temperature-sensitive nanogels that show optimal drug loading and release characteristics because of their capacity to swell and contract stand out the most. For instance, pH-responsive nanogels are created through the use of polymers with deionizable functional groups in the synthesis process Yadav et al. [15].
Because of their small particle size, nanogels offer additional benefits over other drug delivery technologies, such as nanocarriers, in addition to the ones already listed. Nanogels allow for the inhibition of an immunogenic response since they are inert in both the blood and the aqueous milieu. While the latter prevents toxicity and adverse effects brought on by the aggregation of nanomaterials, drug delivery via nanogels enhances biocompatibility and biodegradability. The polymeric network’s functional groups and the medication’s easy integration allow for a higher degree of drug payload. But even with a lowcost production technique, the solvent and surfactant removal can end up being costly Yadav et al. [15] and Jain et al., 2019).
Fast Dissolving Drug Delivery Systems
In subject-specific literature, FDTs are also referred to as porous tablets, quick melting/disintegrating tablets, or dispersible tablets. After being moistened by the salvia, the dissolution or disintegration occurs within a minute without the need for additional liquid or mastication throughout the administration procedure. The first pass metabolism is avoided by prompt absorption of the released medication, allowing for a direct entry into the systemic circulation. In this method, a more effective substitute for traditional oral dosage forms is offered, especially for patients who are bedridden or experiencing nausea and vomiting. The invention of fast dissolving oral films (FDOFs) was prompted by a number of environmental factors, including costly packaging, poor formulas that result in disagreeable flavors, friability, and challenging handling during manufacturing and shipping Heer et al. [16]; Bala et al. 2013 and Irfan et al. [17].
Oral solid dose forms that are most commonly used include tablets and capsules. These are still problematic even though they have many advantages over other delivery routes, such as self-medication, painlessness, and exact dose. The main issue with syrups and other liquid orals is appropriate dosing, even if tablets and capsules might be difficult for elderly, young, and dysphagic patients to swallow and fear suffocation. A unique oral medicine delivery technique is also required in exceptional instances such kinetosis, allergic shocks, or just not having access to water. During the 1970s, rapid dissolving medication delivery devices were primarily created to address swallowing issues. Approximately twenty-five years later, the FDA authorized Zydis ODT, the first fast-dissolving tablet (FDT) containing the antihistamine loratadine. Bhattarai & Gupta [18] (Table 4).

In terms of object qualities like shape and thickness, oral strips are made up of a collection of flat, beautiful films that resemble postage stamps. Depending on the substance and dose level integrated, the size might vary from 1 to 20 cm2. A single dose up to 30 mg is possible (Bala et al., 2013). An optimal film should possess the following properties: good physicochemical capabilities along with flexibility, elasticity, and softness Karki et al. [19]. Fast dissolving films have been the subject of numerous further studies that assess them as innovative drug carriers for various medications, highlighting their significance as novel drug delivery methods which is shown in Table 5.

Osmotic Drug Delivery Systems
A continuous release of the bioactive component at a predetermined rate over a specified, prolonged period of time with controlled and repeatable kinetics is made possible by the creation of controlled drug delivery systems. Osmotic drug delivery systems (ODDSs) are independent of physiological factors, in contrast to conventional controlled dosage forms like matrix systems or reservoir systems, which depend on pH value, GIT motility, and the presence of food. (Patra et al., 2013). Osmotic devices are the most reliable of the many pharmaceutical attempts to create a long-acting pharmacological form for a single daily delivery. The catalyst for the measured release of the API is osmotic pressure. It is feasible to provide medication orally or parenterally; however, there are two types of gastrointestinal therapeutic systems: implantable pumps and oral osmotic pumps. (Sharma et al., 2018). The net motion of water across a semi-permeable membrane caused by the difference in osmotic pressure across this barrier is the usual definition of osmosis. The membrane’s selectivity permits only water to pass through while blocking the entry of most ions and solute molecules. Osmotic pressure-which is created when liquid from the surrounding environment seeps through-controls the release of bioactive chemicals from osmotic devices. Additionally, the degree of medication release is strongly correlated with the core osmotic pressure. Drug release from ODDSs is primarily influenced by soluble, osmotic, delivery orifice dimension, and membrane characteristics. Achieving a constant osmotic pressure gradient between the inner and outer compartment while keeping the osmotic agent in the compartment saturated is crucial. (Patra et al., 2013) Theeuws created the fundamental osmotic pump in the 1970s, which is the most basic type of osmotic pump. The concept comprises a core enclosed by a semi-permeable membrane with one or several delivery pores as seen in Figure 14.
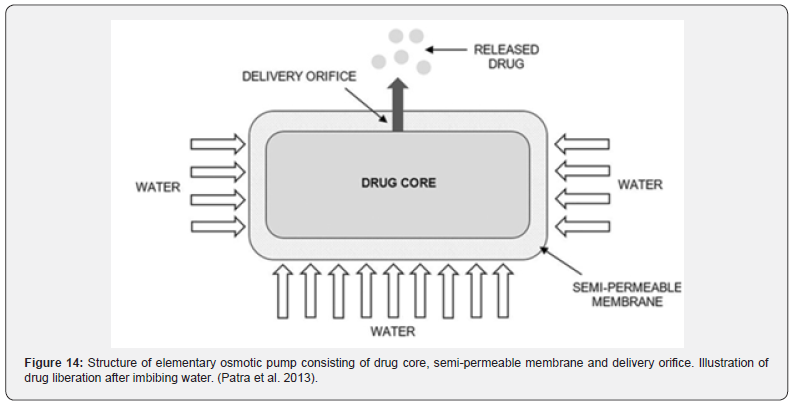
There are three main components in osmotic systems: the drug, the osmotic agent and the semi-permeable membrane. The ideal selection of API should exhibit a brief biological life about 3–6 h. Osmotic agents, or respectively osmogents, are either of organic or inorganic nature, such as sodium chloride, sodium sulphate, and methylcellulose. Semi-permeable membranes have been used in the pharmaceutical industry previously. Good candidates for ODDSs are those with a high potency and are suitable for long-term treatment, especially drugs to treat chronic diseases. Additionally, the API should show a water-solubility within 50–300 mg/L for an optimized drug release Patel & Parikh [20]; Sharma et al. 2018 (Table 6).

In addition to the benefits already discussed, such as the delivery system’s independence from physiological conditions in the gastrointestinal tract, it also has the advantage of a zero-order release following a primary retardation, a fully predictable and programmable drug release rate, and the potential for delayed or pulsed drug delivery. Additionally, this well-researched and defined administration technique improves absorption, lessens side effects, and is appropriate for long-term therapy. Still, there are a few limitations to take into account, such as high costs, poorly made films that cause dose dumping, the size of the delivery orifice, the effect of food intake, the lack of retrieval therapy options, and a rapid onset of tolerance. Sowjanya et al. [21].
Vesicular Drug Delivery Systems
Vesicular systems are highly organized assemblies of one or more concentrical lipid bilayers that are created when amphiphilic building materials come into contact with water. Phospholipids, cholesterol, and non-ionic surfactants are often utilized ingredients in the formulation. Furthermore, there is a diverse range of amphiphilic constituents. According to the form, size, construction, lamellarity, and encapsulation capacity all have a significant impact on efficacy. Because hydrophilic and lipophilic medicines can be entrapped in the aqueous core of the bilayer, respectively, vesicular drug delivery systems (VDDSs) are advantageous over conventional dosage forms. In addition, there are benefits such as enhanced bioavailability, particularly for medications that dissolve slowly, delayed metabolization, extended systemic circulation, and decreased toxicity. Notwithstanding these advantages, VDDDs continue to face a number of disadvantages related to drug loading capacity and drug leakage during in vivo transportation, preservation, and manufacturing (Namdeo et al. 2014) (Figure 15).
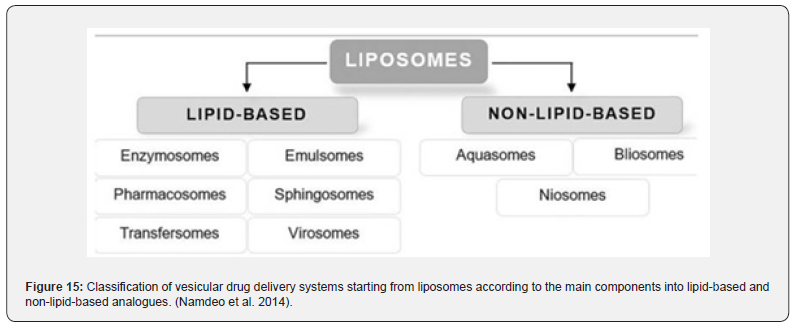
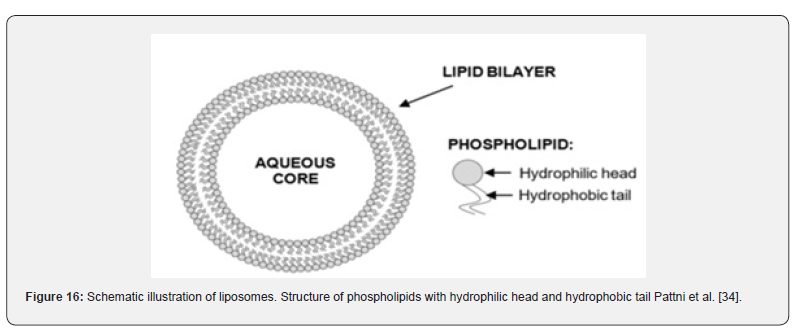

Liposomes
Liposomes are self-assembling, globular blisters made up of one or more concentric lipid bilayers, which can range in size from a few micrometres to an aqueous core. As phospholipids are the primary constituent, membrane formation in watery conditions is highly probable. Shown in Figure 16 (Table 7).
Non-lipid-based analogue
Niosomes: Since they are similar to liposomes in terms of structure and physical characteristics but have a slightly different composition, noisome are thought to be an alternative. Niosomes are made with non-ionic surfactants since the lipids in the original vesicular drug carriers had a high vulnerability to damage and were expensive. The key distinction is reduced costs and improved chemical and physical stability. Non-ionic surfactants selfassemble to create the bilayer structure that characterizes this system. Adhering to the same classification scheme as liposomes, niosomes can be divided into three groups: small, large, and multilamellar vesicles. To further stiffen the bilayer and prevent medication leakage, cholesterol can be added to the formation. Niosomes have the following advantages over conventional liposomal formulations and other DDSs: a broad range of immunogenic, biocompatible, and biodegradable surfactants; multiple routes of administration, such as oral, parenteral, ocular, and topical routes with improved bioavailability; and osmotic activity and stability.
Now that they have taken the cosmetics business by storm, researchers are trying to figure out if niosomes have any further commercial potential. Niosomes’ potential for regulated delivery of clarithromycin was recently investigated by Asthana et al.The niosomal formulation has been found to have both improved bioavailability and sustained and extended drug administration. For sustained administration, aceclofenac-loaded niosomal gels were designed and assessed in a different study that was presented by Fathalla et al. Also examined were the effects of cholesterol, aceclofenac concentration, and non-ionic surfactant extent on encapsulation efficacy. Aceclofenac is an anti-inflammatory medication used to treat rheumatoid arthritis and osteoarthritis; however, its short biological lifespan and poor therapeutic index limit its use. For the preparation, the reverse phase evaporation method was employed. Several methods, such as Fourier-transform infrared spectroscopy, differential scanning calorimetry, optical microscopy, and TEM, were used to characterize the formulations. Selected niosomal formulations containing aceclofenac were added to various gel bases, such as HPMC, PEG 600, and ALG, and their potential for in vitro drug release and skin penetration was examined. The results demonstrated a spherical form with an enhanced drug encapsulation effectiveness and a specific inner watery core of niosomes. Compared to gel formulations supplied with free medication, the niosomal gels offered a prolonged drug. (Ag Seleci et al., 2016).
Lipid-based analogues
Transfersomes: The term “transfersome” refers to a highly elastic, pliable vesicle that may pass through holes even smaller than its own. The notion was initially developed in the 1990s. In addition to phospholipids, the primary ingredients in the formulation of transfersomes are edge activators like span 60 or tween 80. These single chain surfactants have the effect of destabilizing lipid bilayers, which increases their malleability and makes them especially good for penetrating the skin [37-40]. The thin film hydration method and the modified hand shaking method, also known as the lipid film hydration method, are frequently used procedures for the manufacture of transferosomes. For the following unique properties, transfersomes are thought to be beneficial in topical and systemic drug administration. On the one hand, transfersomes have a high encapsulation efficacy, capable of encapsulating up to 90% of medicines with varying molecular weights and solubilities. Additionally, because to the depot function, medication release is gradually enabled while protecting the API from biodegradation and breakdown. In terms of production, a simple large-scale expansion is achievable. A tendency toward oxidative degradation, a range in the purity of phospholipids from natural origin, and an expensive production process are some of the drawbacks that transfersomes still have despite these advantages (Sachan et al., 2013; Sarmah, 2013).
Because asenapine maeleate (AM) has a prolonged hepatic metabolism, it is not recommended to take it orally for the antipsychotic treatment of schizophrenia and bipolar disorder. In a recent work, Shreya et al. [41]. investigated the possibility of using nano-transfersomes to distribute AM transdermally. The study’s objective was to increase skin penetration and, by a combination of chemical and transfersomal approaches, enhance bioavailability. The thin-film hydration method was used to create the transfersomes. The physicochemical characterisation comprised surface shape, vesicle size, zeta potential, incorporation efficacy, and polydispersity index. A study on the in vitro skin permeation of transfersomes loaded with AM was carried out, and various chemical enhancers were employed to augment transdermal transportation. (Shreya et al., 2016).
Ethosomes: Ethosomes Like transferosomes, ethosomes have a faster rate of permeability and a higher transdermal flow, which helps increase penetration through the stratum corneum barrier. These spherical lipid blisters, which are mostly made of phospholipids, ethanol, and water, are an example of the second generation of new vesicular drug carriers. The key characteristic that sets them apart from liposomes is their high alcohol content, which can reach up to 45%. This allows for a reduction in size and elasticity while using the same preparation technique [42- 44]. The amount of transdermal penetration and the natural skin barrier’s penetration are modified in order to reach deeper tissues and have a systemic effect. Additional adjuvants included in the ethosomal formulation include gel markers for longer residence times or cholesterol to enhance stability. Generally speaking, the methods for getting ready.
Jain and colleagues have developed and assessed ethosomal hydrogels for transdermal administration of diclofenac. The aim of this study was to enhance the drug’s anti-inflammatory properties while also understanding the relationship between formulation parameters, physicochemical characteristics, and penetration flux. Different strategies for improving transdermal drug absorption via various drug carriers and permeation enhancers are troublesome since they cause long-lasting skin damage and painful, valuable treatments. Liposomal control formulations and ethosomes loaded with diclofenac were produced using the rotary evaporation process. The results of the in vitro skin penetration investigation and physicochemical characterization showed the impact of the interaction of variable elements, particularly size and flexibility, as well as controllability through its manipulation (Jain et al., 2015).
ADDS of Herbal Formulation
In recent decades, there has been a lot of research and development focused on ADDS for herbal treatments. The unique carriers should preferably satisfy two requirements. First and foremost, the medication must be taken during treatment at a rate that is determined by the body’s needs. Second, it must deliver the active component of the herbal remedy to the affected area. The existing conventional or extended-release dosage formulations are unable to meet any of these requirements. Numerous cuttingedge herbal formulations, such as polymeric nanoparticles, nanocapsules, liposomes, phytosomes, nanoemulsions, microspheres, transferosomes, and ethosomes, have made use of bioactive and plant extracts. The global advanced drug delivery systems market was estimated to be worth $134.3 billion in 2008; by 2009, that amount is anticipated to increase to $139 billion. The second-largest market share is possessed by products with sustained release. Sustained release of medications with a short half-life can result in less frequent dosage, better compliance, and reduced variation in blood and plasma levels, all of which can enhance the consistency of the therapeutic impact.
Future Perspectives and Critical Point of View
Today’s drug delivery technologies make it possible for the medicine to be embodied in innovative delivery systems, which facilitates a number of medicinal and commercial benefits. Numerous innovative delivery mechanisms, ranging from quick dissolving to nanoparticulate, have been created and reviewed. Additionally, a number of ways for regulated drug administration to a specific target-site have been investigated. Still, there are a number of difficulties. Polymers are essential for both creating drug carriers and navigating drug liberation, which is one of the constants in drug delivery systems. The availability of appropriate biocompatible polymers limits advancement, particularly in the realm of nanotechnology. The need for polymers with specific physical and biological properties has led to an ongoing interest in the synthesis of novel polymers. For this reason, a broad range of synthetic and natural biodegradable polymers have been investigated for their potential to provide targeted drug delivery and prolonged drug liberation. Only a few of them have been proven to be biocompatible thus far. Conventional methods are easier to scale up from a manufacturing standpoint, but they may become less precise in monitoring particle properties. While topdown approaches would provide size and form regulation, they are limited to specific drug delivery methods. Various alternative methods have been proposed to overcome the constraints associated with insulin administration in the management of diabetes mellitus. Insulin delivery rates can be controlled via glucose modulation, allowing for self-regulated medication administration. Creating a delivery method that mimics the in vivo pattern of insulin release is the main obstacle.
In summary, the requirement for new materials with the qualities of biodegradability, biocompatibility, and low toxicity will be satisfied in the future, and when combined with advanced manufacturing methods, they will offer substantial benefits in drug delivery. The distribution of pharmaceuticals in the future will undoubtedly present greater challenges, and the field of pharmaceutic research has an incredibly difficult task.
Concluding Remarks
The development of novel drug delivery systems plays a leading role in pharmaceutical sector nowadays. Conventional dosage forms such as tablets, capsules and emulsions are limited by several drawbacks. To address these shortcomings of traditional drug delivery systems research mainly focused on improving bioavailability and patient compliance while reducing toxicity and side effects. A variety of drug delivery technologies has been developed and evaluated, and numerous strategies for a controlled and targeted delivery have been explored. The performance of the drug in terms of bioavailability, safety and efficacy can be positively changed by alteration of an already existing drug into a novel delivery technology. Although oral administration is one of the most widely used and patient-complies with methods, oral bioavailability is frequently restricted due to inadequate permeability, high first pass metabolism, or poor water solubility. Through the creation of fast-dissolving formulations that included FDTs and subsequently FDOFs, scientists adopted a different approach to conventional oral dosage forms.
Its unique qualities, like its simplicity of administration, speedy disintegration, and 60-second half-life, make it a potentially effective delivery method, particularly for elderly, juvenile, and dysphasic patients who have difficulty swallowing pills or capsules. Self-emulsifying formulations are becoming more and more common for preclinical investigations because over 40% of newly discovered medications have poor water solubility, which results in poor bioavailability and missed dose proportionality. Because of its design, SEDDS, SMEDDS, and SNEDDS can be administered orally without going through the dissolution stage, and their first pass effect indicates higher levels of bioavailability. Since their invention approximately 25 years ago, osmotic medication devices have advanced significantly based on the use of osmosis as the driving mechanism. The delivery of various API by ODDSs is controlled and independent of physiological circumstances. This is achieved by modifying formulation variables such as the gist’s dissolubility and osmotic pressure, membrane properties, and orifice size. Among the fascinating areas of medicine delivery, nanotechnology has recently grown at an impressive rate. Both organic and inorganic nanoparticles can be loaded with a wide range of active agents and have their size and shape controlled. They can also be functionalized for targeted distribution and triggered release and can be made with a vast variety of materials and formulation variables. Moreover, lipoidal vesicular systems like ethosomes, transfersomes, and liposomes, as well as nonlipoidal vesicular systems like niosomes, can be used to effectively deliver drugs to a particular target region. By doing this, harmful or unwanted impacts on other sites can be avoided.
Medicinal plants contain bioactive compounds such as alkaloids, flavonoids, and terpenoids, which offer therapeutic benefits like antioxidant, anti-inflammatory, and anticancer effects. However, their clinical use is often limited by poor bioavailability, low solubility, and rapid metabolism Bellah et al. [22,23]; Howlader et al. 2012; Momin et al. [24-26]; Sm Faysal Bellah et al. [27,28]; Faysal Bellah et al. [29]. Drug delivery systems (DDS), particularly nanoparticles, can enhance the efficacy of plant-based medicines. Nanoparticles offer several advantages, including improved solubility, stability, and controlled release of bioactive compounds. They also enable targeted delivery, ensuring that therapeutic agents reach specific tissues, such as cancer cells, while minimizing side effects. By integrating medicinal plant compounds with nanoparticles, DDS can enhance the bioavailability and precision of treatment, overcoming limitations like the blood-brain barrier for neurological conditions. This combination of plant-derived bioactive compounds with advanced drug delivery systems opens new avenues for more effective, targeted therapies. Thus, nanoparticles and DDS significantly improve the therapeutic potential of medicinal plants in modern medicine Bellah et al. [22], Momin et al. [24]; Ashrafudoulla et al. [30,31]; Rezaul et al. [32] and Ferdous et. Al., 2018).
In conclusion, All things considered, medication delivery system evolution has advanced significantly and will continue to expand at a remarkable rate. The use of drug molecules in advanced drug delivery systems offers numerous therapeutic and economic advantages. In the distribution of both new and current pharmaceuticals, it is clear that new avenues have been explored and doors opened. However, there are several issues that need to be addressed more thoroughly, such as the translation to clinical application, high production costs, and little drug payload. But there’s always potential for improvement, and before being authorized for use in people, any newly created drug delivery system must undergo a comprehensive investigation and characterization.
Acknowledgment
We thank members of our groups for insightful discussions during this study.
References
- Patel SM, Patel RP, Prajapati BG (2012) Solubility enhancement of benfotiamine, a lipid derivative of thiamine by solid dispersion technique. J Pharm Bioallied Sci 4(Suppl 1): S104.
- Liechty WB, Kryscio DR, Slaughter BV, Peppas NA (2010) Polymers for drug delivery systems. Annual Review of Chemical and Biomolecular Engineering 1: 149-173.
- Patel R, Shah D, Prajapti BG, Patel M (2010) Overview of industrial filtration technology and its applications. Indian Journal of Science and Technology 3(10):1121-1127.
- Ulbrich K, Holá K, Šubr V, Bakandritsos A, Tuček J (2016) Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chemical Reviews 116(9): 5338-5431.
- Vugmeyster Y, Xu X, Theil FP, Khawli LA, Leach MW (2012) Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World Journal of Biological Chemistry 3(4): 73-92.
- Glassman PM, Muzykantov VR (2019) Pharmacokinetic and pharmacodynamic properties of drug delivery systems. The Journal of Pharmacology and Experimental Therapeutics 370(3): 570-580.
- Paul KM, Fathima SCM, Nair (2017) Intra nasal in situ gelling system of lamotrigine using ion activated mucoadhesive polymer, Open Med Chem J p: 11.
- Bialik M, Kuras M, Sobczak M, Oledzka E (2021) Achievements in thermosensitive gelling systems for rectal administration, thermosensitive gelling systems 55: 851-858.
- Wang H, Huang Y (2020) Combination therapy based on nano codelivery for overcoming cancer drug resistance, Med. Drug Discovery 16: 340-355.
- Silva F, Sitia L, Allevi R, Bonizzi A, Sevieri M, et al. (2021) Combined method to remove endotoxins from protein nanocages for drug delivery applications: the case of human ferritin, pharmaceutics 130: 202-229.
- Rani K, Paliwal S (2014) A review on targeted drug delivery: its entire focus on advanced therapeutics and diagnostics, Scholars. J Appl Med Sci 2: 328-331.
- Thakor AS, Gambhir SS (2013) Nano oncology: the future of cancer diagnosis and therapy. CA Cancer J Clin 63: 395-418.
- Frank LA, Contri R, Beck RCR, Pohlmann AR, Guterres SS (2015) Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 7: 623-639.
- Lee SC, Kwon IK, Park K (2013) Hydrogels for delivery of bioactive agents: a historical perspective. Adv Drug Deliv Rev 65: 17-20.
- Yadav HKS, Anwar N, Halabi A, Alsalloum GA (2017) Nanogels as Novel Drug delivery Systems - a Review Properties of Nanogels Keywords : Introduction Advantages of Nanogels. Insight Pharma Res 1 :1-
- Heer D, Aggarwal G, Kumar SLH (2013) Recent trends of fast dissolving drug delivery system - an overview of formulation technology. Pharmacophore 4: 1-9.
- Irfan M, Rabel S, Bukhtar Q, Qadir MI, Jabeen F (2016) Orally disintegrating films: a modern expansion in drug delivery system. Saudi Pharm J 24: 537-546.
- Bhattarai M, Gupta AK (2016) Fast Dissolving Oral Films: a Novel Trend to Oral Drug delivery System. Sunsari Tech Coll J 2: 58-68.
- Karki S, Kim H, Na SJ, Shin D, Jo K (2016) Thin films as an emerging platform for drug delivery. Asian J Pharm Sci 11: 559-574.
- Patel HJ, Parikh VP (2017) An overview of Osmotic Drug delivery System: an update review. Int J Bioassays 6: 54-26.
- Sowjanya M, Venkata Prasada Rao Ch, Srinivasa Babu PK (2017) Osmotic drug delivery systems: a review. Pharma Times 7: 1-9.
- Sm Faysal B, Momin Mohammad AM, Islam Mohammad M, Khan Mohammad S, Anisuzzaman S (2012) Development and validation of method for determination of Esomeprazole by HPLC. International Research Journal of Pharmacy 3(7): 227-232.
- Mohammad Abdul MM, Sm Faysal B, Afrina A, Kaniz FU, Kaiser H (2012) Phytochemical Screening and Cytotoxicity Potential of Ethanolic Extracts of Senna siamea Leaves. Journal of Pharmaceutical Science and Research 4(8): 1877-1879.
- Momin Mohammad AM, Anisuzzaman SMd, Begum Anjuman A, Bellah Sm F, Islam Md. Mostaharul (2013) Development and validation of method for determination of Ciclesonide INN (Micronized) by HPLC. International Research Journal of Pharmacy 4(7): 55-59.
- Mohammad Abdul MM, Sm Faysal B, Mizanur RK, Iqubal HR, Mustafizur R (2014) Phytopharmacological evaluation of ethanolic extract of Feronia limonia leaves. American Journal of Scientific and Industrial Research 4(5): 468-472.
- Mohammad Abdul MM, Sm Faysal B, Sarder Mohammad RR, Ahmed AR, Gazi Mohammad MM, et al. (2014) Phytopharmacological evaluation of ethanolic extract of Sida cordifolia L. roots. Asian Pacific Journal of Tropical Biomedicine 4(1): 18-24.
- Sm Faysal B, Firoj Ahmed AAR, Shahid IZ, Moazzem HSM (2012) Preliminary phytochemical, anti-bacterial, analgesics, anti-diarrhoeal and cytotoxic activity of methanolic extract of Polyalthia suberosa leaves. International Journal of Pharmaceutical Science and Research 3(5): 1322-1326.
- Sm Faysal B, Iqubal Hossain R, Saker Billah SM, Shaikh ER, Murshid GMM (2015) Evaluation of antibacterial and antidiarrhoeal activity of ethanolic extract of Feronia limonia Leaves. The Pharma Innovation Journal 3(11): 50-54.
- Sm Faysal B, Nur I, Rezaul K, Masudur R, Samima N (2016) Evaluation of the cytotoxic, analgesic, antidiarrheal and phytochemical properties of Hygrophila spinosa ( Anders) whole plant. Journal of Basic and Clinical Physiology and Pharmacology 28(2): 185-190.
- Ashrafudoulla Md, Sayed Sakib F, Rezaul K, Sm Faysal B (2015) Hypoglycemic complications with diabetes mellitus management: the predominant adverse drug reaction presenting to the accident and emergency Patient of Birdem Hospital Dhaka, Bangladesh. The Pharma Innovation Journal 4(10): 97-100.
- Ashrafudoulla, Sm Faysal B, Feroz A, Sayed Sakib F, Abdullah Hil K (2016) Phytochemical screening of Solanum nigrum L, S. myriacanthus Dunal, Solanum melongena and Averrhoa bilimbi in Bangladesh. Journal of Medicinal Plants Studies 4(1): 35-38.
- Rezaul K, Abdul A, Sm Faysal B, Nasir U, Sultan M (2018) Comparative free radical scavenging, thrombolytic, cytotoxic, antimicrobial and analgesic activities of different parts of Centella asiatica (Apiaceae) Britsh Journal of Pharmaceuticals and Medical Research 3(2): 885-890.
- Razowanul F, Shohel H, Ashrafudolla, Sm Faysal B (2018) Evaluation of Antioxidant, Analgesic and Antidiarrheal activities of methanolic extract of Litsea monopetala () leaves. Clinical Pharmacology & Biopharmaceutics 7(3): 1-6.
- Sun Y, Lau SY, Lim ZW, Chang SC, Ghadessy F (2022) Phase-separating peptides for direct cytosolic delivery and redox-activated release of macromolecular therapeutics. Nat Chem 14(3): 274-283.
- Martinelli C, Pucci C, Ciofani G (2019) Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng 3: 507-514.
- Pattni BS, Chupin VV, Torchilin VP (2015) New Developments in Liposomal Drug delivery. Chem 115: 109-121.
- Web-1 S. Controlled drug delivery systems: Current status and future directions Molecules.
- Web-1 drugs delivery system (SMEDDS)-challenges and road ahead.
- Web-1 Biomolecules. Photothrombotic using erythrocyte-based particles.
- Web-1 Injectable in situ gelling delivery system for the treatment of jawbone infections.
- Gonzalez-Gomez A, Sanz B, Roman JS, Goya GF, Hernandez R (2017) combined drug delivery and magnetic hyperthermia: from preparation to in vitro studies, Carbohydr. Polym 157: 361-370.
- Kannan (2016) Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells, an improved nanomedicine strategy. Sci Rep 6: 340-353.
- Web-1 Nano oncology: the future of cancer diagnosis and therapy.
- Sariful Islam H, Muhammad Shahdaat BS, Maizbha UA, Abdul Kader M, Zubair Khalid L, et al. (2012) Characterization of Chemical Groups and Study of Antioxidant, Antidiarrhoeal, Antimicrobial and Cytotoxic activities of ethanolic extract of Diospyros blancoi (Family: Ebenaceae) Leaves. Journal of Pharmacy Research 5(6): 3050-3052.






























