Abstract
Alzheimer’s disease (AD) is a serious public health concern that is becoming more widespread worldwide. The pancreas is the primary source of the endocrine hormone insulin, which is becoming more widely acknowledged for its significance in controlling brain activity. Insulin receptors are abundant in the hippocampus, one of the initial regions of the brain to be impacted by AD. Since all cell types in the central nervous system (CNS) have insulin and insulin-like growth factor-1 receptors, insulin plays a critical role in brain function. In the brain, insulin regulates glucose metabolism, supports cognition, and promotes neuronal growth. Additionally, insulin from the bloodstream can pass through the blood-brain barrier. Insulin resistance and other disturbances in insulin signalling may hasten the ageing process of the brain, alter neuroplasticity, and possibly even cause neurodegeneration. The AKT pathway, which governs metabolic consequences, and the MAPK pathway, which governs gene expression, cell proliferation, and survival, are two significant insulin signalling pathways. This review aims to investigate the role of insulin in Alzheimer’s disease.
Keywords: Alzheimer’s disease; Insulin resistance; Oxidative stress; Neuroinflammation; Type 2 diabetes mellitus
Abbreviations:AD: Alzheimer’s Disease; CNS: Central Nervous System; IRs: Insulin Receptors; CSF: Cerebrospinal Fluid; BBB: Blood–Brain Barrier; T2DM: Type 2 Diabetes Mellitus; PSD: Postsynaptic Density; LTD: Long-Term Depression; LTP: Long-Term Potentiation; NMDA: N-Methyl-D-Aspartate; MAP: Microtubule-Associated Protein; NFTs: Neurofibrillary Tangles; APP: Amyloid Precursor Protein; IDE: Insulin-Degrading Enzyme; RNS: Reactive Nitrogen Species; ROS: Reactive Oxygen Species; BACE1: Boosting Beta-Secretase-1; ApoE: Apolipoprotein E
Introduction
With the discovery of insulin receptors (IRs) and the subsequent demonstration of insulin’s hypoglycaemic effects in 1916, the crucial function of IRs in controlling glucose metabolism in peripheral tissues has been determined [1,2]. By boosting the translocation of glucose transporters, including GLUT 4, to the plasma membrane, insulin enhances glucose transport into cells and facilitates glucose utilisation in peripheral tissues. In addition to glucose metabolism, insulin affects protein synthesis, cell division, and growth. Historically, it was believed that the brain was an insulin-insensitive organ, and that IR function was mostly peripheral. This idea derived from the observation that circulating insulin levels do not appear to have an impact on whole-brain glucose absorption [3]. However, studies conducted in the last 20 years have revealed a special function for insulin in the brain. Even though plasma has much higher levels of insulin than cerebrospinal fluid (CSF), relationships between the two have been found, suggesting that pancreatic secretion is the primary source of brain insulin [2]. Through capillary endothelial cells, insulin penetrates the blood–brain barrier (BBB) in a selective, saturable, receptor-dependent manner [4].
The brain uses transcytosis to move insulin–insulin receptor complexes, which are created when insulin attaches to insulin receptors (IRs). Many variables, including astrocyte activity, obesity, diabetes, inflammation, high-fat diets, and circulating triglyceride levels, can affect this process [5]. Moreover, research on animals indicates that CSF insulin levels increase following meals and fall during fasting. The BBB may be disturbed by insulin resistance, changing its permeability and leading to cerebrovascular dysfunction, which can affect synaptic development and cognitive function. It is thought that some areas of the brain, like the choroid plexus and hypothalamus, enable peripheral insulin to enter the central nervous system (CNS) more quickly [3]. There is ongoing controversy about whether the brain synthesizes insulin de novo. Human CSF has been found to contain C-peptide, an intermediate product of insulin manufacture, and certain brain areas in rats and mice have been found to contain insulin mRNA. Additionally, post-mortem investigations have documented the presence of insulin mRNA in human brain tissue, specifically in the hypothalamus and hippocampus [4]. Neurodegenerative illnesses, such as Alzheimer’s disease (AD), are significantly influenced by the hippocampus, a crucial learning and memory centre [6].
Insulin receptors are abundantly distributed in the hippocampus, and there is considerable interest in understanding how insulin affects memory through hippocampal mechanisms [7]. Intranasal delivery of insulin has been shown to effectively target CNS regions, delivering biologically active insulin. Despite the fact that the exact mechanisms are still unknown, a number of recent research have shown that intranasal insulin improves memory impairment in both clinical and animal models. Type 2 diabetes mellitus (T2DM) has become increasingly prevalent due to rising obesity rates and population aging [8]. Importantly, individuals with T2DM are shown to have twice the risk for acquiring cognitive dysfunction in comparison to those without T2DM. Both clinical and animal studies have revealed significant correlations between the pathologies of AD and T2DM, with insulin resistance being one of the most critical connections [9]. This review focusses on the connection between insulin and AD, examining the molecular connections between insulin resistance and AD and providing an overview of the ways in which insulin affects memory. These insights could inform the discovery of novel diagnostic and treatment approaches in the future.
Role of Insulin in Brain
Although there are many insulin receptors in the brain, the olfactory bulb, hypothalamus, hippocampus, cerebral cortex, and cerebellum have the highest densities. The synapse is a crucial location for specialised insulin signalling since these receptors are primarily found on neurons and have high levels of expression at presynaptic axon terminals and inside the postsynaptic density (PSD) [10,11]. The short isoform A (IR-A) is the one that is most frequently expressed in the brain.
Insulin and brain glucose metabolism
Insulin-regulated transporters like GLUT4 and GLUT8, which are found in areas including the hippocampus, amygdala, hypothalamus, and basal forebrain, co-express GLUT3, which is the primary mechanism by which neurons absorb glucose [2,12]. By promoting GLUT4 expression and its transfer to the plasma membrane through the AKT route, insulin improves glucose uptake and utilisation, which is crucial for memory and cognition, especially during times of high energy consumption like learning [13]. Animal studies reveal that insulin-driven GLUT4 translocation in the hippocampus boosts sugar breakdown and spatial memory, while in astrocytes, it promotes glucose uptake and glycogen storage. GLUT8, predominantly expressed in the hippocampus, supports neuronal glucose homeostasis. In addition to its central role, brain insulin signaling affects peripheral metabolism by regulating hepatic glucose production, lipolysis, amino acid catabolism, and triglyceride secretion via hypothalamic pathways influencing vagal and sympathetic output [2].
Insulin and cognition
The role of insulin receptors (IRs) in learning and memory is shown by the substantial amount of IRs in brain regions such the frontal cortex, entorhinal cortex, and hippocampus. Insulin injection has been shown to enhance memory in both people and animals. This effect may be due to its involvement in hippocampal synaptic plasticity, which includes long-term depression (LTD) and long-term potentiation (LTP), both of which are essential for memory and learning [14]. Through the ERK1/2 and PI3-K pathways, insulin affects the expression of N-methyl-D-aspartate (NMDA) receptors, which aids in synaptic remodelling and neural plasticity [50–52]. It has been demonstrated that spatial learning increases IR mRNA in the hippocampus, specifically in the dentate gyrus and CA1 area, which promotes tyrosine phosphorylation and insulin receptor accumulation [15]. These findings suggest that learning can modulate IR expression and signalling in the hippocampus. Beyond cognition, insulin signalling impacts mood and emotional regulation. Elevated IRs in limbic regions may affect mood, reward, and motivation. Insulin treatment has been shown to improve mood, reduce anxiety, and enhance object memory in animal models. However, a decrease in hypothalamic IRs leads to behaviour that resemble depression and anxiety [2]. It has also been demonstrated that long-term intranasal insulin treatment improves human memory [16]. Taken together, our results demonstrate that the hippocampus insulin receptor is an important target for emotional and cognitive functions [16].
Insulin resistance and AD
Insulin resistance is simply “reduced sensitivity of body tissues to insulin” in people with type 2 diabetes. Likewise, the incapacity of brain cells to react to insulin efficiently is known as brain insulin resistance. This resistance can arise from several factors, including downregulation of insulin receptors, impaired insulin binding, or dysfunction in the insulin signal transmission [17]. At the molecular level, insulin resistance in the brain might show up as reduced discharge of neurotransmitters or disturbed neuroplasticity, which can result in deficits in mood, cognition, and metabolism regulation [2]. Alzheimer’s disease (AD) patients’ brains have metabolic abnormalities that are quite similar to those observed in type 2 diabetes. This similarity has led to the hypothesis that AD could be a brain-specific form of type 2 diabetes, often referred to as “type 3 diabetes” [10].
Role of Tau Protein in AD and Insulin Resistance
Tau is a microtubule-associated protein (MAP) that stabilizes microtubules, influencing processes like cell morphogenesis, division, intracellular trafficking, and synaptic function [3]. It is regulated by phosphorylation, with over 85 potential phosphorylation sites, and its activity is influenced by insulin and IGF signalling. In Alzheimer’s disease (AD), Tau is hyperphosphorylated—up to three times higher than normal— with over 40 phosphorylation sites identified in AD brains, 28 of which are unique to the disease [18]. Hyperphosphorylation disrupts Tau’s ability to bind microtubules, leading to aggregation into neurofibrillary tangles (NFTs), a characteristic feature of AD and tauopathies. This process impairs cellular functions, including organelle transport, and contributes to oxidative stress, apoptosis, and mitochondrial dysfunction [3]. Several kinases, including GSK3β, AMPK, and ERK, regulate Tau phosphorylation, while insulin resistance exacerbates the process through impaired PI3-K/AKT signalling, increased GSK3β activity, and reduced Tau O-GlcNAcylation due to diminished glucose metabolism [10]. Oxidative stress, a consequence of insulin resistance, further activates GSK3β, enhancing Tau pathology. According to research on animals, Tau controls insulin signalling in the brain. Deletion of Tau affects hippocampus insulin responsiveness and energy metabolism through changes in IRS-1 and PTEN activity. These changes link Tau dysfunction to brain insulin resistance, cognitive decline, and metabolic impairments in AD [19].
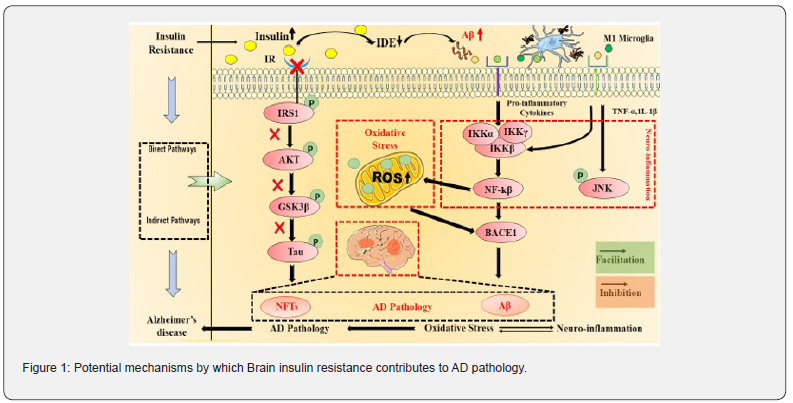
Role of amyloid precursor protein (APP) and Amyloid-β (Aβ) in AD and insulin resistance
Neuronal synapses contain a transmembrane protein called amyloid precursor protein (APP), which is implicated in iron export, synapse development, and brain plasticity. APP is processed via two different routes: the non-amyloidogenic (non-plaque-forming) and amyloidogenic (plaque-forming) pathways. Insulin promotes non-amyloidogenic processing, reducing pathological amyloid-β (Aβ) accumulation, while impaired insulin signalling increases Aβ formation by reducing its degradation via insulin-degrading enzyme (IDE) [3,10]. Soluble APPα (sAPPα) and a non-toxic fragment are released when α-secretase cleaves APP in the non-amyloidogenic pathway. In contrast, the amyloidogenic pathway involves β- and γ-secretase activity, producing Aβ peptides (primarily Aβ40 and Aβ42) that aggregate into neurotoxic plaques. Aβ42, particularly toxic, is associated to synaptic disruption, neuronal dysfunction, and cell death. Mutations in APP, presenilins (PS1/PS2), or Apolipoprotein E - (ApoE-ε4) allele increase Aβ42 production, contributing to early-onset Alzheimer’s disease (AD). Sporadic AD features similar Aβ accumulation but involves other poorly understood factors. Insulin regulates APP metabolism, influencing Aβ balance. Reduced insulin signaling elevates Aβ levels, promoting plaque formation, oxidative stress, and Tau hyperphosphorylation. IDE, which degrades insulin and Aβ, is essential for this process. Reduced IDE expression in AD brains leads to hyperinsulinemia, glucose intolerance, and increased Aβ levels, contributing to neurodegeneration and insulin resistance [1]. Aβ affects insulin signalling by inhibiting PI3-K/AKT signalling, competing with insulin for receptor binding, and activating GSK-3β, which increases Tau hyperphosphorylation and APP processing. This feedback loop exacerbates AD pathology. Targeting Aβ synthesis, enhancing its clearance, or restoring insulin signalling may offer therapeutic strategies for mitigating AD progression and associated insulin resistance.
Role of inflammation in AD and insulin resistance
Neuroinflammation is a fundamental contribution to Alzheimer’s disease (AD) pathogenesis, caused by the deposition of amyloid-β (Aβ), which causes the production of proinflammatory cytokines (e.g., IL-1, IL-6, TNF-α, and TGF-β) by chronically activated glial cells. Elevated levels of these cytokines are commonly detected in the cerebrospinal fluid of AD patients, indicating that they are involved in neurodegeneration [20]. The brain’s inherent immune mediators, microglia and astrocytes, play a key role in neuroinflammation. Reactive oxygen species (ROS), reactive nitrogen species (RNS), and inflammatory mediators are released by activated microglia, leading to oxidative stress, synaptic dysfunction, and neuronal damage. Chronic inflammation further exacerbates insulin resistance and AD progression [21].
Glycosylation of proteins and lipids produces advanced glycation end products (AGEs), which build up into neurofibrillary tangles and amyloid plaques. When AGEs engage with their receptor (RAGE), nuclear factor kappa beta (NF-κB) is activated, thereby turning up inflammatory pathways. Additionally, RAGE binds Aβ, promoting amyloidogenic APP processing by boosting beta-secretase-1 (BACE1) production and allowing its passage over the blood-brain barrier (BBB). These mechanisms perpetuate inflammation and neurodegeneration in AD. Peripheral insulin resistance links metabolic dysfunction to AD, increasing AGEs, stimulating IDE expression, and impairing insulin signaling. Insulin typically suppresses inflammation and supports synaptic function via the PI3K-AKT-mTOR pathway. Dysregulated signaling, influenced by apolipoprotein E (ApoE) and other factors, amplifies neuroinflammatory cascades, contributing to cognitive decline in AD [22,23]. Thus, inflammation, Aβ accumulation, and insulin resistance form a feedback loop driving AD pathology and progression, highlighting potential therapeutic targets.
Insulin resistance and oxidative stress in AD
Through increased GSK-3β activation, disruption of lipid and carbohydrate metabolism, and impairment of mitochondrial and cell survival, insulin resistance exacerbates oxidative stress. Additionally, it impacts brain function by decreasing the expression of neurotrophins and choline acetyltransferase and is linked to increased levels of Aβ42 and phosphorylated Tau. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced as a result of oxidative stress, which is brought on by conditions like hypoxia and ischaemia. These species harm proteins, lipids, and nucleic acids, which contributes to neurodegeneration, especially in the initial phases of Alzheimer’s disease (AD) [24]. The pathophysiology of AD is mostly influenced by malfunctioning mitochondria, which impairs energy metabolism and neurological functioning by producing less ATP and more ROS. Enzyme activity involved in glycolysis, the citric acid cycle, and the respiratory chain is decreased in AD brains, leading to synapse loss and neurodegeneration [25]. While oxidative stress is an early feature in AD, mitochondrial enzyme activity increases before amyloid plaque formation. However, Aβ oligomers may exacerbate mitochondrial dysfunction by reducing cytochrome oxidase activity and increasing ROS production.
Conclusion
The onset and growth of Alzheimer’s disease (AD) are significantly influenced by brain insulin resistance, mainly through oxidative stress and the production of amyloid-beta (Aβ42) and Tau protein phosphorylation, which affect memory, cognitive function, and mitochondrial function. Additionally, AD is also associated with a number of other pathologies. Often called “diabetes of the brain,” or type 3 diabetes, AD is treated with medications that are comparable to those for diabetes. In conclusion, the interplay between insulin resistance, oxidative stress, Aβ accumulation, Tau hyperphosphorylation, and neuroinflammation forms a complex network that drives the neurodegenerative processes in Alzheimer’s disease. Addressing these interconnected factors may offer therapeutic avenues for mitigating the cognitive and metabolic impairments associated with AD.
Acknowledgment
The authors are grateful to UIPS, Panjab University, Chandigarh, India for providing the facilities.
References
- Liu Q, Wang Z, Cao J, Dong Y, Chen Y (2022) The Role of Insulin Signalling in Hippocampal-Related Diseases: A Focus on Alzheimer's Disease. Int J Mol Sci 23(22): 14417.
- Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A (2019) Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol 234(6): 8152-8161.
- Sędzikowska A, Szablewski L (2021) Insulin and Insulin Resistance in Alzheimer's Disease. Int J Mol Sci 22(18): 9987.
- Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 4(3): 168-181.
- Gray SM, Aylor KW, Barrett EJ (2017) Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia 60(8): 1512-1521.
- Park KH, Noh Y, Choi EJ, Kim H, Chun S (2017) Functional Connectivity of the Hippocampus in Early- and vs. Late-Onset Alzheimer's Disease. J Clin Neurol 13(4): 387-393.
- Gralle M, Labrecque S, Salesse C, De Koninck P (2021) Spatial dynamics of the insulin receptor in living neurons. J Neurochem 156(1): 88-105.
- Zimmet P, Shi Z, El-Osta A, Ji L (2018) Epidemic T2DM, early development and epigenetics: implications of the Chinese Famine. Nat Rev Endocrinol 14(12): 738-746.
- Antal B, McMahon LP, Sultan SF, Lithen A (2022) Type 2 diabetes mellitus accelerates brain aging and cognitive decline: Complementary findings from UK Biobank and meta-analyses. Elife 11: e73138.
- Kim AB, Arvanitakis Z (2023) Insulin resistance, cognition, and Alzheimer disease. Obesity 31(6): 1486-1498.
- Carvalho C, Cardoso SM, Correia SC, Moreira PI (2019) Tortuous Paths of Insulin Signalling and Mitochondria in Alzheimer's Disease. Adv Exp Med Biol 1128: 161-183.
- Neth BJ, Craft S (2017) Insulin Resistance and Alzheimer's Disease: Bioenergetic Linkages. Front Aging Neurosci 9: 345.
- Surguchov A (2020) Caveolin: A New Link Between Diabetes and AD. Cell Mol Neurobiol 40(7): 1059-1066.
- Gratuze M, Joly-Amado A, Vieau D, Buée L, Blum D (2018) Mutual Relationship between Tau and Central Insulin Signalling: Consequences for AD and Tauopathies? Neuroendocrinology 107(2): 181-195.
- Spinelli M, Fusco S, Mainardi M, Scala F, Natale F (2017) Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat Commun 8(1): 2009.
- Folch J, Ettcheto M, Busquets O, Sánchez-López E, Castro-Torres RD (2018) The Implication of the Brain Insulin Receptor in Late Onset Alzheimer's Disease Dementia. Pharmaceuticals (Basel) 11(1): 11.
- Burillo J, Marqués P, Jiménez B, González-Blanco C, Benito M (2021) Insulin Resistance and Diabetes Mellitus in Alzheimer's Disease. Cells 10(5):1236.
- Rad SK, Arya A, Karimian H, Madhavan P, Rizwan F (2018) Mechanism involved in insulin resistance via accumulation of β-amyloid and neurofibrillary tangles: link between type 2 diabetes and Alzheimer's disease. Drug Des Devel Ther 12: 3999-4021.
- Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A (2017) Tau deletion promotes brain insulin resistance. J Exp Med 214(8): 2257-2269.
- Gupta S, Nair A, Jhawat V, Mustaq N, Sharma A (2020) Unwinding Complexities of Diabetic Alzheimer by Potent Novel Molecules. Am J Alzheimers Dis Other Demen 35:1533317520937542.
- Femminella GD, Livingston NR, Raza S, Van der Doef T, Frangou E (2021) Does insulin resistance influence neurodegeneration in non-diabetic Alzheimer's subjects? Alzheimers Res Ther 13(1): 47.
- Zhou YL, Du YF, Du H, Shao P (2017) Insulin resistance in Alzheimer's disease (AD) mouse intestinal macrophages is mediated by activation of JNK. Eur Rev Med Pharmacol Sci 21(8): 1787-1794.
- Robbins J, Busquets O, Tong M, De la Monte SM (2020) Dysregulation of Insulin-Linked Metabolic Pathways in Alzheimer's Disease: Co-Factor Role of Apolipoprotein E ɛ4. J Alzheimers Dis Rep 4(1): 479-493.
- Dewanjee S, Chakraborty P, Bhattacharya H, Chacko L, Singh B (2022) Altered glucose metabolism in Alzheimer's disease: Role of mitochondrial dysfunction and oxidative stress. Free Radic Biol Med 193(Pt1): 134-157.
- Marrano N, Biondi G, Borrelli A, Rella M, Zambetta T (2023) Type 2 Diabetes and Alzheimer's Disease: The Emerging Role of Cellular Lipotoxicity. Biomolecules 13(1): 183.






























