The Lipolytic Effect of Cassia obtusifolia L Seeds on 3T3-L1 Adipocytes
Meei-Cho Lai1, Yu-Ru Lee2, Ming-Hua Tsai3 and Jung-Ren Chen3*
1Department of Pharmacy, Tajen University, Pingtung 90741, Taiwan
2Graduate Institute of Environmental Management, Tajen University, Pingtung 90741, Taiwan
3Department of Medical Science & Biotechnology, I-Shou University, Kaohsiung 82445, Taiwan
Submission:April 10, 2024; Published:April 19, 2024
*Corresponding author:Jung-Ren Chen, Department of Medical Science & Biotechnology, I-Shou University, Kaohsiung 82445, Taiwan Email: jrchen@isu.edu.tw
How to cite this article: Meei-Cho L, Yu-Ru L, Ming-Hua T, Jung-Ren C. The Lipolytic Effect of Cassia obtusifolia L Seeds on 3T3-L1 Adipocytes. The simplified normative rules NBP in Italy. An Review. J of Pharmacol & Clin Res. 2024; 10(2): 555783. DOI: 10.19080/JPCR.2024.10.555783
Abstract
Cassia obtusifolia L of the Leguminosae family, a commonly used traditional Chinese medicine, has been employed for its therapeutic properties, including heat-clearing, vision improvement, blood nourishment, and intestinal moistening. It is believed to possess pharmacological effects such as hepatoprotection, blood pressure reduction, lipid-lowering, immune enhancement, anti-aging, Alzheimer's disease improvement, and laxative effects. Lipid-lowering refers to the ability of certain substances to reduce fat accumulation or promote fat metabolism. However, the current scientific research on the lipid-lowering effects of C. obtusifolia L is limited. In this study, we subjected C. obtusifolia L to different processing methods and evaluated the extracts using 3T3-L1 adipocytes. Statistical analysis was conducted to compare the differences between the water and alcohol extracts of the raw and processed samples. The impact of different processing methods on fat metabolism and synthesis was assessed. The results revealed that the vinegar-processed alcohol extract of C. obtusifolia L exhibited the most prominent inhibitory effect on fat accumulation. This extract contained abundant flavonoid compounds, such as C. obtusifolia L flavones and C. obtusifolia L flavonoid glycosides. These compounds are believed to stimulate fat metabolism and inhibit fat synthesis, thereby contributing to the reduction of intra-organ fat accumulation. Further research is warranted to elucidate the underlying mechanisms and evaluate the potential therapeutic applications of these seeds in weight management.
Keywords:Lipolytic; Adipocytes; Cassia obtusifolia; Leguminosae family; Alzheimer’s disease; Glycosides
Abbreviations:WHO: World Health Organization; HPLC: High-Performance Liquid Chromatography; RO: Reverse Osmosis; DMSO: Dimethyl Sulfoxide; DMEM: Dulbecco’s Modified Eagle Medium; FBS: Fetal Bovine Serum
Introduction
Overweight and obesity are global health issues caused by an energy imbalance, with energy intake exceeding energy expenditure. Obesity is a major risk factor for various other diseases, including type 2 diabetes, hypertension, heart disease, and stroke. Recognized as a chronic condition in modern medicine, obesity affects the functioning of internal organs and leads to numerous complications. Currently, traditional Chinese medicine offers limited pharmacological treatments for overweight and obesity, while the use of Western medicine may come with side effects [1]. The World Health Organization (WHO) has shown increasing interest in traditional medicine, including herbal medicine, in recent years, and the Chinese community highly values the application of herbal medicine in daily life and disease treatment. C. obtusifolia L, listed as a superior herb in the Shen Nong's Herbal Classic, is a commonly used traditional Chinese medicine and a valuable resource as it possesses hepatoprotective, immune-enhancing, and anti-aging properties. In the past few decades, it has been used for benefits of or care for the eyes, liver protection and the treatment of overweight and other metabolic disorders such as hyperlipidemia and diabetes [2]. To investigate the potential lipid-lowering effect of C. obtusifolia L and its contribution to weight loss, this study examines the lipid-lowering properties of C. obtusifolia L using four different processing methods.
These methods include raw and processed samples, utilizing water and alcohol extracts. Additionally, the study evaluates the effects of these processing methods on lipid-lowering by assessing their impact on 3T3-L1 adipocytes. Furthermore, High-Performance Liquid Chromatography (HPLC) is employed to measure the changes in composition of the key component, chrysophanol, in raw and processed cassia seeds. By correlating the processing methods with the lipid-lowering effects observed in 3T3-L1 adipocytes, this study aims to provide insights into the potential of C. obtusifolia L as a weight-loss agent. C. obtusifolia L, listed as a superior herb in the Shen Nong's Herbal Classic, is a commonly used traditional Chinese medicine in clinical practice. It is mainly produced in provinces in China, such as Anhui, Guangxi, Sichuan, Zhejiang, and Guangdong, where it is cultivated in various regions. The seeds are harvested in autumn, dried, and then removed from the pods for use. The Compendium of Materia Medica describes its therapeutic effects as follows: "It is used for treating blurred vision, excessive ocular discharge, red and white membranes, redness and pain in the eyes, and prolonged use improves visual acuity." Its taste is described as sweet, bitter, salty, and neutral, and it enters the liver meridian. C. obtusifolia L refers to the dried mature seeds of the annual herbaceous plants C. obtusifolia L or C. tora L. They are rhomboid in shape, resembling horse hooves, slightly pointed at one end, and truncated at the other, with a yellow-green surface and a slightly bitter taste [3].
Fresh C. obtusifolia L seeds contain components such as chrysophanol, emodin, aloe-emodin, rhein, mucilage, ketones, and polysaccharides, which find extensive applications in medicine and the food industry. C. obtusifolia L extracts can increase the expression of cell hormones, thereby stimulating immune responses in T cells. Modern medical research has shown that C. obtusifolia L possesses hepatoprotective, blood pressure-lowering, lipid-lowering, immune-enhancing, anti-aging, Alzheimer's disease-improving, laxative, anti-platelet aggregation, and anti-obesity properties, as well as the ability to relieve constipation. C. obtusifolia L holds great potential for research and product development in medicinal and health-related fields [4,5]. The processing of traditional Chinese medicine is a pharmaceutical technique based on the theory of Chinese medicine. It involves processing medicinal herbs into medicinal materials according to different requirements such as medical treatment, formulation, preparation, and storage, as well as the properties of the herbs themselves. Traditional Chinese medicine processing technique is used when processing herbs into medicinal slices. In this study, C. obtusifolia L were subjected to four types of processing techniques, namely Stir-fried processed, Alcohol processed, vinegar processed, and salt processed, followed by extraction for experimentation [6,7]. 3T3-L1 murine preadipocytes are commonly used for analyzing preadipocyte differentiation, also known as adipogenesis.
Few cell types can undergo adipocyte differentiation, with 3T3-L1 cells being a well-established model for studying adipogenesis [8,9]. 3T3-L1 fibroblasts differentiate into lipid-rich adipocytes, making them one of the most commonly used in vitro models in adipocyte biology research. This model is essential for studying adipogenesis, lipid metabolism, and hormone action. The preadipose cell line 3T3-L1 cells were isolated and expanded from Swiss 3T3 cells based on their ability to accumulate lipids [8-11]. Under normal conditions, 3T3-L1 preadipocytes exhibit a fibroblast-like phenotype. However, when treated with differentiation medium, these cells accumulate lipid droplets and acquire the characteristics of mature adipocytes. The process of adipocyte differentiation takes approximately two weeks, and differentiation can be confirmed through staining [12]. The preadipocyte cell line differentiates into mature adipocytes by treatment with various differentiation agents after growth arrest, including synthetic glucocorticoids like dexamethasone and the phosphodiesterase inhibitor 1-methyl-3-isobutylxanthine (IBMX) [8,10]. The most common differentiation protocol involves a combination of all three drugs [13-15]. The preadipocytes undergo cell cycle regulation and are then exposed to differentiation medium containing insulin, dexamethasone, and IBMX for two days. Subsequently, the cells are re-exposed to insulin for two days and then returned to normal growth medium [16]. The early response to differentiation medium involves upregulation of genes that drive adipogenesis, including C/EBP and PPAR subtypes [17,18]. Activation of the adipogenic gene expression program increases glucose uptake and triglyceride synthesis, with noticeable lipid accumulation observed in the cells approximately four days after the initial exposure to the differentiation medium [11].
Materials & Methods
Preparation Methods of C. obtusifolia L Seeds
The C. obtusifolia L seeds were prepared using different methods, including stir-frying, alcohol treatment, vinegar treatment, and salt treatment, following the traditional Chinese medicine processing techniques.
Stir-fried processed
Clean and dry 200g of C. obtusifolia L seeds. Place them in an infrared oven and heat at 180°C for 10-30 minutes. Remove from the oven and let them cool.
Alcohol processed
Add 200g of C. obtusifolia L seeds to 20ml of yellow alcohol and let them soak for one day. On the next day, remove the seeds and place them in an infrared oven. Heat at 180°C for 10-30 minutes. Remove from the oven and let them cool.
Vinegar processed
Add 200g of C. obtusifolia L seeds to 20 ml of vinegar and let them soak for one day. On the next day, remove the seeds and place them in an infrared oven. Heat at 180°C for 10-30 minutes. Remove from the oven and let them cool.
Salt processed
Add 200g of C. obtusifolia L seeds to 20ml of 2% saline solution and let them soak for one day. On the next day, remove the seeds and place them in an infrared oven. Heat at 180°C for 10-30 minutes. Remove from the oven and let them cool.
Extraction Method of C. obtusifolia L Seeds
The four different processed C. obtusifolia L seeds were separately soaked in 300ml of reverse osmosis (RO) water and 95% ethanol for 24 hours. Ultrasonic oscillation was performed for 3 hours, and the extract was then filtered under vacuum. The filtered extract was subjected to vacuum concentration and freeze-dried to obtain crude extract, which was stored at -20°C for further use. The C. obtusifolia L seed extract powder was weighed and dissolved in RO water and dimethyl sulfoxide (DMSO) to prepare different concentrations. The solution was then filtered through a 0.22µm filter for later use.
Evaluation of the Lipolytic Effect of C. obtusifolia L Seeds on 3T3-L1 Adipocytes
3T3-L1 Cell Culture
3T3-L1 murine preadipocytes were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium supplemented with 10% fetal bovine serum (FBS) and maintained in a 5% CO2, 37°C incubator. After one or two days, when the cells reached 70-80% confluency, 2000 cells/well were seeded in 96-well culture plates [19].
Cell Viability Assay
Cell viability was determined using the 3‐(4, 5‐dimethylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide (MTT) assay, which measures cell metabolic activity [20]. Eight different C. obtusifolia L seed extracts were prepared by dissolving the extract powder in DMEM medium to achieve concentrations of 6.25, 3.15, 1.6, and 0.8 ppm. The solutions were filtered through a 0.22 µm filter. The culture medium in the 96-well plates containing 3T3-L1 cells was replaced with the respective concentrations of the seed extracts and incubated for 72 hours. Then, 100 µL of 5mg/ml MTT solution was added to each well and incubated for 1-4 hours. The formazan crystals formed by viable cells were dissolved in dimethyl sulfoxide (DMSO), and the absorbance at 570nm was measured using a spectrophotometer to determine cell viability. The cell viability percentage was calculated using the formula:
[OD570(sample)/ OD570(control)] × 100%
Adipocyte Differentiation Inhibition Assay
Adipocyte differentiation inhibition was assessed using the Oil Red O lipid staining and quantification method. Oil Red O dye binds to intracellular lipid droplets, staining them red [21-25]. Cells with higher levels of accumulated lipid droplets indicate a higher degree of adipocyte differentiation. The culture medium in the 6-well plates containing 3T3-L1 cells was replaced with DMEM medium supplemented with 10% fetal bovine serum for four days of proliferation. When the cells reached confluency, external differentiation agents (10 μg/ml insulin, 0.5 mM IBMX, and 1 μM dexamethasone) were added to induce cell differentiation. This day was designated as day 0 of cell differentiation. Fresh medium with insulin was replaced every two days. The eight different C. obtusifolia L seed extracts were prepared by dissolving the extract powder in DMEM medium to achieve a concentration of 0.8 ppm and filtered through a 0.22 µm filter. The culture medium in the 6-well plates was replaced with the 0.8 ppm seed extract solutions and incubated for 24 hours. The cells were fixed with 10% formaldehyde (2400 μl) and kept in fresh formaldehyde for up to two days. After removal of formaldehyde, the cells were washed with isopropanol and stained with Oil Red O dye. The stained cells were photographed, and to extract the lipid content, the cells were incubated with isopropanol. The absorbance at 520nm was measured using a spectrophotometer, and the absorbance value was proportional to the concentration of lipids. The percentage of adipocyte differentiation inhibition was calculated using the formula:
[OD520(sample)/ OD520(control)] × 100% [26, 27]
High-performance liquid chromatography (HPLC) Analysis
High-performance liquid chromatography was performed to determine the composition of C. obtusifolia L seeds extract on a HITACHI 2695 under the following conditions: Column: ODS, 5 μm, (4.6 × 250 mm), Mobile phase A: Acetonitrile, Mobile phase B: 0.1% H3PO4, Detection: HITACHI 5430 Detector UV at 254 nm, Flow rate: 1 mL/min Injection volume: 10 μL [28,29]. Approximately 0.5 g of the sample powder was accurately weighed and placed in a conical flask. To this, precisely 50 mL of methanol was added and the weight was determined. The mixture was heated under reflux for 3 hours, allowed to cool, and reweighed. The weight loss was compensated by adding methanol, and the solution was thoroughly mixed. The resulting solution was filtered and the filtrate was concentrated to dryness. Then, 30 mL of 10% hydrochloric acid was added and the mixture was heated under reflux for 1 hour. After cooling, the ethyl acetate layer was extracted four times with 30 mL portions.
The combined ethyl acetate layers were concentrated to dryness. The residue was dissolved in methanol and transferred to a 25 mL volumetric flask. Methanol was added to the flask up to the mark, and the solution was mixed, filtered, and used as the test solution. The test solution was further filtered through a 0.45 μm membrane filter before subjecting it to HPLC analysis. Standard chrysophanol was accurately weighed and dissolved in 100% methanol to prepare a series of solutions with different concentrations. The solutions were filtered through a 0.45 μm membrane filter to obtain the standard curve solution. Then, 10 μL of each solution was injected for High-performance liquid chromatography (HPLC) analysis. The peak area or height of the standard solutions was used for linear regression to construct a calibration curve and determine the equation and correlation coefficient of the calibration curve.
Statistical Analysis
The significance of the differences was by the unpaired Student's t-test. Statistical analyses were performed using Prism statistical software (Prism 7.0a; GraphPad Software Inc., San Diego, CA, USA). The significance level was set at p ≤ 0.05. A p-value less than 0.05 was considered statistically significant, indicating meaningful differences among the groups.
Results
Cell Viability(MTT)Assay
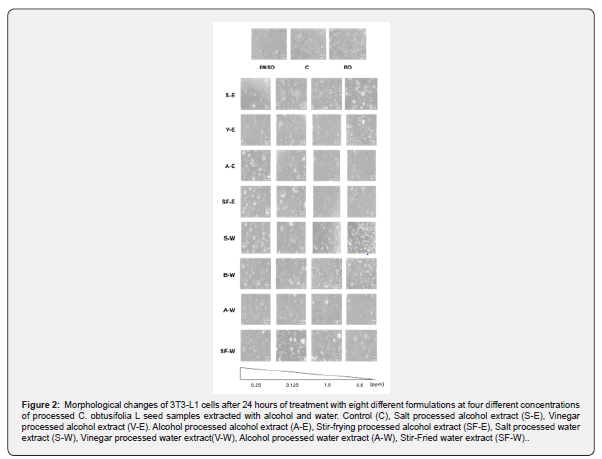
The cell viability assay was performed on 3T3-L1 cells using eight different samples, consisting of four different processed C. obtusifolia L seed samples extracted with either water or alcohol, at various concentrations. Among these samples, the lowest concentration of 0.8 ppm exhibited the highest cell viability, indicating lower cytotoxicity towards 3T3-L1 cells (Figure 1). The morphological changes of the 3T3-L1 cells were examined under a phase-contrast microscope after 24 hours of treatment with eight different formulations at four different concentrations of raw and processed C. obtusifolia L seed samples extracted with alcohol and water. The results suggest that the 3T3-L1 cells treated with 0.8 ppm of the extracts appeared healthy and least deleterious (Figure 2). Concentrations at 0.8 ppm of the extracts were chosen in the subsequent quantification experiment. For quantification, Oil Red O dye was used to stain intracellular lipid droplets, which appear red. Cells with a higher abundance of lipid droplets indicate a greater degree of adipocyte differentiation.
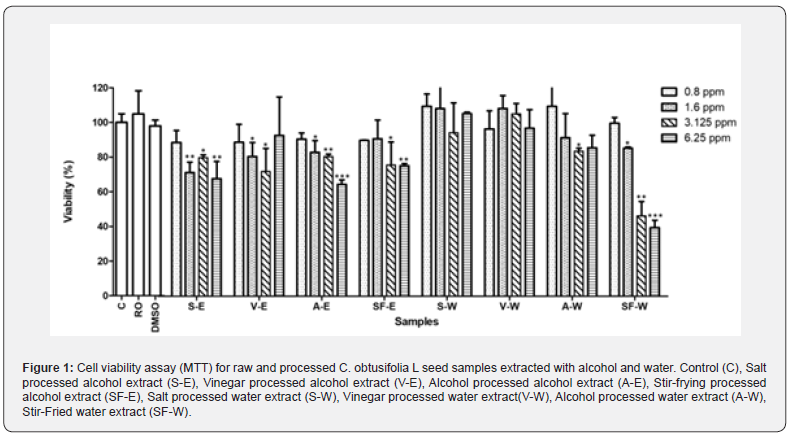
Inhibitory effects of the eight different formulations on 3T3-L1 cells
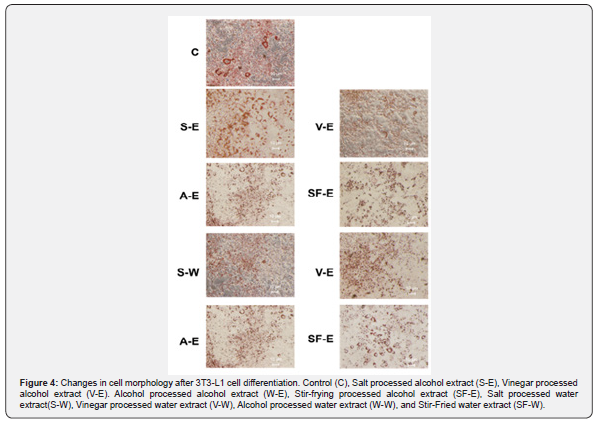
To evaluate the inhibitory effects of the eight different formulations on 3T3-L1 cells, cells were plated in 6-well plates containing DMEM supplemented with 10% fetal bovine serum for four days to allow proliferation. Upon reaching confluency, external differentiation agents (10 μg/ml insulin, 0.5 mM IBMX, and 1 μM dexamethasone) were added to induce cell differentiation. The culture medium in the 6-well plates was then replaced with the eight different 0.8 ppm C. obtusifolia L seed extract solutions and incubated for 24 hours. Based on the cell inhibition rate test conducted on 3T3-L1 cells using the eight different samples at a concentration of 0.8 ppm, it can be concluded that two of the alcohol extracts (salt processed alcohol extract (S-E) and vinegar processed alcohol extract (V-E)) exhibited greater inhibitory effects on 3T3-L1 cells compared to most of the water extracts, except for salt processed water extract (S-W), which showed comparable effects to salt processed alcohol extract (S-E). Overall, the vinegar processed alcohol extract showed the highest inhibitory effect (Figure 3).
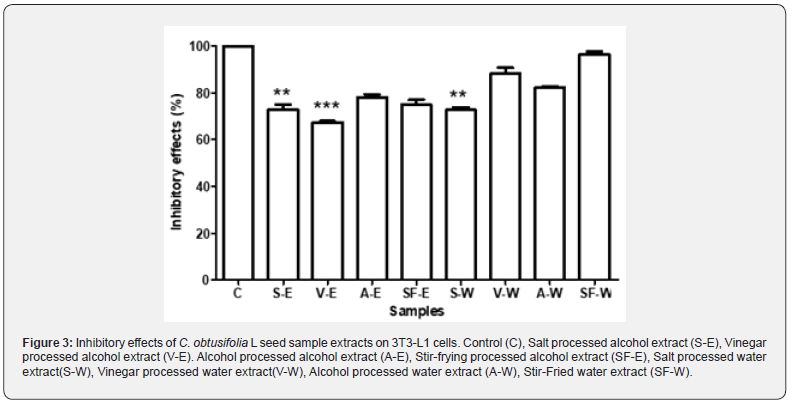
Adipocyte Differentiation Inhibition Assay
The differentiation inhibitory effects of C. obtusifolia L seed samples extracts on 3T3-L1 cells were investigated after 24 hours of treatment with eight different formulations at four different concentrations, using the Oil Red O lipid staining and quantification method. In the control group, visible red lipid droplets were observed, indicating the accumulation of lipid droplets around the adipocytes, while in the treatment groups, the formation of red lipid droplets was absent (Figure 4).
High-performance liquid chromatography (HPLC) Analysis
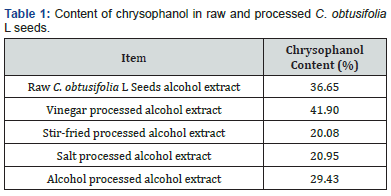
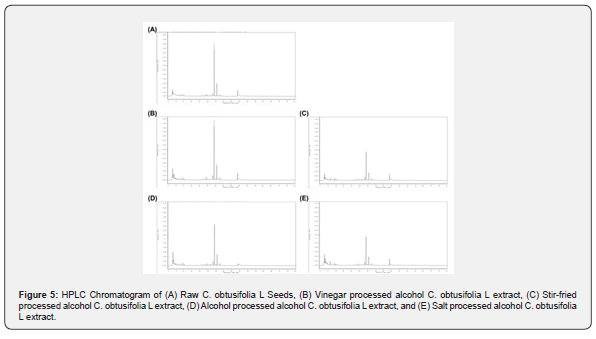
Chrysophanol has been shown to exhibit lipid lowering properties in rats in 2013, and Chrysophanol has also been shown to lower cholesterol and triglyceride levels in zebrafish, as well as increase the frequency of peristalsis [30]. HPLC was conducted to analyze the composition of C. obtusifolia L seed extracts (Figure 5). The results revealed the content of chrysophanol (%) in raw and processed C. obtusifolia L seeds (Table 1) as follows: Raw C. obtusifolia L had a chrysophanol content of 36.65%, vinegar processed alcohol extract had a chrysophanol content of 41.90%, stir-fried processed alcohol extract had a chrysophanol content of 20.08%, alcohol processed alcohol extract had a chrysophanol content of 29.43%, and salt processed alcohol extract C. obtusifolia L had a chrysophanol content of 20.95%,.
Discussion
obtusifolia L are classified as a superior herb in the Shen Nong's Herbal Classic and are commonly used in clinical practice of traditional Chinese medicine. According to the Compendium of Materia Medica, they are documented to possess various activities such as improving eyesight, reducing blood pressure, protecting the liver, enhancing immunity, and anti-aging effects. The preparation of traditional Chinese medicine involves a series of processing and treatments of Chinese herbal medicines to enhance their efficacy, reduce toxicity, or alter the properties of the drugs. Generally, traditional Chinese medicines undergo preparation before use. From a modern scientific perspective, the basic principle of preparation is to alter the chemical composition of medicinal herbs through processing, thereby changing the effects of the drugs. Different drugs have different mechanisms of preparation, and studying the preparation process can help understand the mechanisms of action of traditional Chinese medicine staining [31-34]. Obesity, a condition characterized by an excess accumulation of body fat, arises from a complex interplay of factors predominantly centered around an energy imbalance: excessive caloric intake compared to insufficient energy expenditure. This imbalance is often perpetuated by modern dietary habits favoring calorie-dense foods, sedentary lifestyles, genetic predispositions, and environmental factors influencing food availability and activity levels.
Over time, the surplus energy is stored in the form of adipose tissue, leading to weight gain and subsequent obesity. Furthermore, obesity is not just a matter of excess weight but also a precursor to various metabolic syndromes, including type 2 diabetes and high blood lipids, which significantly increase the risk of cardiovascular diseases and other health complications. In addressing obesity and its associated metabolic disorders, interventions targeting lipid-lowering actions play a pivotal role. Lipid-lowering actions encompass a range of strategies aimed at reducing fat accumulation or enhancing fat metabolism, including lifestyle modifications such as dietary changes and increased physical activity, pharmacological treatments like statins and fibrates, and the utilization of dietary supplements. By focusing on improving metabolic health, lowering blood lipid levels, and mitigating cardiovascular risks associated with obesity, these interventions foster long-term well-being and reduce the burden of obesity-related health complications on individuals and healthcare systems alike. In our study, we investigated the effects of processing C. obtusifolia L, a herb known for its medicinal and edible properties, using different adjuvants (alcohol, salt, and vinegar) and various processing methods (Stir-frying processing, vinegar processing, salt processing, and alcohol processing). Subsequently, we extracted the processed herbs using both reverse osmosis (RO) water and alcohol. To evaluate the outcomes, we employed the 3T3-L1 cell line and conducted tests to assess the impact of these processing methods on fat metabolism and synthesis. Statistical analysis was then employed to compare the differences between the water extraction and alcohol extraction groups for both raw and processed products.
Through this comprehensive approach, we aimed to determine the extent to which different processing techniques and extraction methods influence the metabolic properties of C. obtusifolia L, shedding light on its potential applications in promoting or inhibiting fat metabolism and synthesis. The findings of our study demonstrate that vinegar-processed alcohol extract of C. obtusifolia L exhibited the most potent inhibitory effect on fat accumulation, providing scientific validation for the herb's potential application in weight reduction therapies, in line with evidence-based medical practices. Notably, changes in the composition of emodin in C. obtusifolia L were observed before and after processing, with higher emodin content detected after dry frying compared to raw material, while other processing methods led to a decrease in emodin content. However, further evidence is warranted to elucidate the specific lipid-lowering effects of C. obtusifolia L. Moving forward, our focus will be on identifying the active ingredients within its components for further testing, with the ultimate goal of developing processed C. obtusifolia L as a medicinal herb specifically targeted for weight reduction purposes. This research represents a significant step towards harnessing the therapeutic potential of C. obtusifolia L in combating obesity and related metabolic disorders.
References
- Gittelsohn J, Rowan M (2011) Preventing diabetes and obesity in American Indian communities: the potential of environmental interventions. Am J Clin Nutr 93(5): 1179S-1183S.
- Yuan-yuan W, Zhe M, Han-wen S (2011) GC-MS Analysis of Chemical Constituents Fatty Extracted from Cassiae Seed by Ultrasonic Extraction. Journal of Hebei University 31(3): 258.
- Ali MY, Park S, Chang M (2021) Phytochemistry, ethnopharmacological uses, biological activities, and therapeutic applications of Cassia obtusifolia: a comprehensive review. Molecules 26(20): 6252.
- Yun-Choi HS, Kim JH, Takido M (1990) Potential inhibitors of platelet aggregation from plant sources, V. Anthraquinones from seeds of Cassia obtusifolia and related compounds. J Nat Prod 53(3): 630-633.
- Hao Y, Sang Y, Zhao Y (2001) The advancement of the studies on the seeds of Cassia obtusifolia. Chinese Tradit Herb Drugs 32: 858-859.
- Alanazi HH (2023) Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases. Molecules 28(24): 8045.
- Fathifar Z (2023) New approaches in developing medicinal herbs databases. Database p: 110.
- Hua Y (2016) Prolonged treatment with 3-isobutyl-1-methylxanthine improves the efficiency of differentiating 3T3-L1 cells into adipocytes. Anal Biochem 507: 18-20.
- Cave E, Crowther NJ (2019) The use of 3T3-L1 murine preadipocytes as a model of adipogenesis. Methods Mol Biol pp: 263-272.
- Miao JY (2000) 1-Methyl-3-isobutylxanthine delays apoptosis induced by deprivation of growth factors in vascular endothelial cells. Acta Pharmacol Sin 21(10): 936-938.
- Zebisch K (2012) Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem 425(1): 88-90.
- Kralisch S (2005) Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J Endocrinol 185(3): R1-R8.
- Pantoja C, Huff JT, Yamamoto KR (2008) Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell 19(10): 4032-4041.
- Katafuchi T, Garbers DL, Albanesi JP (2010) CNP/GC-B system: A new regulator of adipogenesis. Peptides 31(10): 1906-1911.
- Wu XC (2020) Combined use of dbcAMP and IBMX minimizes the damage induced by a long‐term artificial meiotic arrest in mouse germinal vesicle oocytes. Mol Reprod Dev 87(2): 262-273.
- Reul BA (1997) Insulin and insulin-like growth factor I antagonize the stimulation of ob gene expression by dexamethasone in cultured rat adipose tissue. Biochem J 342: 605-610.
- Gold G, Qian RL, Grodsky GM (1988) Insulin biosynthesis in HIT cells: effects of glucose, forskolin, IBMX, and dexamethasone. Diabetes 37(2): 160-165.
- Kanda K (2012) Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim Biophys Acta 1820(4): 461-468.
- Etesami B (2020) Investigation of 3T3-L1 cell differentiation to adipocyte, affected by aqueous seed extract of Phoenix Dactylifera Rep Biochem Mol Biol 9(1): 14.
- Buranaamnuay K (2021) The MTT assay application to measure the viability of spermatozoa: A variety of the assay protocols. Open Vet J 11(2): 251-269.
- Koopman R, Schaart G, Hesselink MK (2001) Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem cell biol 116: 63-68.
- Mehlem A (2013) Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat protoc 8(6): 1149-1154.
- Kraus NA (2016) Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 5(4): 351-358.
- Escorcia W (2018) Quantification of lipid abundance and evaluation of lipid distribution in Caenorhabditis elegans by nile red and oil red O staining. J Vis Ex (133): e57352.
- Lim SH (2021) Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int J Mol Sci 22(21): 11409.
- Boone C (2000) The adipose conversion process: regulation by extracellular and intracellular factors. Reprod Nutr Dev 40(4): 325-358.
- Morrison S, McGee SL (2015) 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte 4(4): 295-302.
- Mao R (2022) Transcriptome and HPLC Analysis Reveal the Regulatory Mechanisms of Aurantio-Obtusin in Space Environment-Induced Senna obtusifolia Lines. Int J Environ Res Public Health 19(2): 898.
- Wu DT (2019) Extraction optimization, structural characterization, and antioxidant activities of polysaccharides from Cassia seed (Cassia obtusifolia). Molecules 24(15): 2817.
- Chen K, Wang CQ, Fan YQ, Xie YS, Yin ZF, et al. (2015) Application of chrysophanol in zebrafish to reduce dietary introduced lipid and its possible mechanism. Int J Clin Exp Med 8(7): 10558-10567.
- Wan M (2023) Ethnopharmacological use, pharmacology, toxicology, phytochemistry, and progress in Chinese crude drug processing of the lateral root of Aconitum carmichaelii Debeaux. (Fuzi): A review. J Ethnopharmacol 30(301): 115838.
- Liou SS (2005) Comparison of the antinociceptive action of crude Fuzei, the root of Aconitum, and its processed products, J Ethnopharmacol 99(3): 379-383.
- Wu X (2018) Seeing the unseen of Chinese herbal medicine processing (Paozhi): advances in new perspectives. Chin Med 13: 1-13.
- Zhu YP (1995) Traditional Chinese herbal medicine, Pharmacy World and Science 17: 103-112.






























