Psoriasis: Concepts of Pathogenesis
Sahoo PK* and Bhumika Kumar
Delhi Institute of Pharmaceutical Sciences and Research University, New Delhi, India
Submission: March 13, 2023; Published: August 10, 2023
*Corresponding author: Sahoo PK, Delhi Institute of Pharmaceutical Sciences and Research University, New Delhi, India. Email id: pksahoo10@rediffmail.com
How to cite this article: Sahoo PK* and Bhumika Kumar. Psoriasis: Concepts of Pathogenesis. J of Pharmacol & Clin Res. 2023; 9(2): 555764. DOI: 10.19080/JPCR.2023.09.555764
Abstract
Psoriasis is a genetic skin or articular disease that develops in the immune system or both. To treat this disease, a diverse team of clinicians with a wide range of expertise is frequently required. Psoriasis causes a variety of issues, including higher incidence, chronicity, and disfigurement, as well as co-morbidities such as psoriatic arthritis. Knowing about the significance of immune function in psoriasis, as well as the relationship between the innate and adaptive immune systems, has led to the treatment of this multifactorial disease that affects millions of people worldwide. In this review, we discuss the pathogenesis of psoriasis as well as potential treatment options. We provide a brief overview of recent advances in psoriasis epidemiology, pathogenesis, and genetics in order to better understand current trends in psoriasis management.
Keywords: Psoriasis; Pathophysiology; Psoriatic Skin; Psoriatic Plaque
Abbreviations: DC: Dendritic Cells; DDC: Dermal Dendritic Cells; IFN: Interferon; PDC: Plasmacytoid Dendritic Cells; TNF: Tumor Necrosis Factor; TH: T helper
Introduction
Psoriasis is a serious genetic skin condition that causes both physical and psychological distress. It is a severe, autoimmune skin condition characterized by systemic proinflammatory stimulation. This affects both men and women equally, with most cases appearing between the second and fourth decades of life [1] Nonetheless, approximately 75% of patients develop psoriasis in their early 30s. Stress, genetic factors and environmental triggers have a crucial role in the disease’s pathogenesis [2,3]. The pathophysiology of the disease is primarily characterized by the stimulation and movement of T cells into the dermis, which results in the release of cytokines (in general, tumor necrosis factor-alpha TNF-alpha) that cause inflammation and accelerated skin cell development. Emotional stress, skin damage, systemic infections, certain medications, and bowel disorders are all potential triggers for psoriasis. Therapeutic agents that either modulate the immune system or redefine the differentiation process of psoriatic keratinocytes are used in its management [4]. Treatment regimens for psoriasis vary depending on the type of psoriasis, position, extent, and degree of disease severity, and include topical agents, phototherapy, systemic agents, that can help control symptoms. The primary objective of this overview is to raise awareness about the nature of this multidimensional disorder, the efficacy of cutting-edge treatment approaches, and the importance of early identification and intensive psoriasis patient care.
Pathogenesis
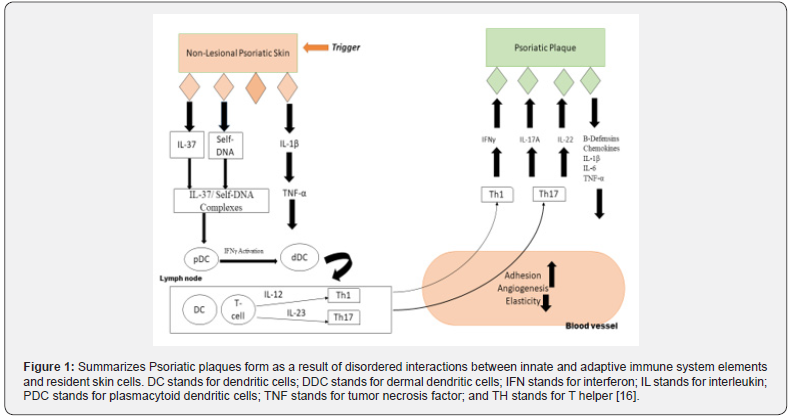
Psoriasis is now widely recognized as an immune-mediated disease. As previously stated, studies of genome-wide correlations found that psoriasis was primarily associated with immune-related genes [5] Psoriatic plaques are thought to be the result of dysregulated connections between innate and adaptive immune system components and native skin cells. (Figure 1) depicts the pathological roadmap of non-lesioned psoriatic skin and the psoriatic plaque epidermis. Because of immune system dysregulation, skin resident cells generate LL-37, which interacts with DNA and stimulates plasmacytoid dendritic cells, further initiating dermal dendritic cells by releasing interferon-gamma. Dendritic cells stimulate T lymphocytes in lymph nodes (T cell) which in turn releases Interleukins (IL), IL-12, and IL-23 and activate T-helper cells (Th1 and Th17, respectively). Once in the bloodstream, these Th-cells release IFN-, IL-17, and IL-22, which cause keratinocytes to modify and proliferate, ultimately resulting in the thicker epidermis as seen in psoriasis [6]. To comprehend how these anti-psoriasis strategies work, knowledge of the disease’s dynamic pathophysiology is required [7–10].
Treatments of Psoriasis
Topical therapeutic approaches are the first-line psoriasis treatment option. In the case of moderate psoriasis, topical agents alone provide relief of up to 80%. For moderate disease management, topical treatments such as glucocorticoids, vitamin D supplements, and combinations of both are usually sufficient. Tacrolimus is a topical calcineurin inhibitor which is used in difficult-to-treat areas such as the intertriginous regions and the face. Corticosteroids that are strong or very effective are preferable to vitamin D3 analogues for scalp treatment [1]. Over the last decade, many biological products have been developed and approved for the treatment of psoriasis. Although only a few head-to-head research studies have been conducted, biological effectiveness appears to be better for short-term treatment than traditional systemic medications [11]. Except for etanercept, a fusion protein, the only approved biologics are monoclonal antibodies. The TNF inhibitors etanercept, adalimumab, and infliximab are approved for the treatment of psoriasis and psoriatic arthritis. TNF inhibitors are frequently used following phototherapy or when traditional systemic therapies are ineffective, intolerable, or contraindicated. Phototherapy is the treatment of choice when local treatments are insufficient, or the affected area of the body exceeds 20%. UVB and UVA lights are used for phototherapy which can be used in conjunction with topical agents like retinoids to improve UVB permeation.
Due to the various side effects associated with it like light hypersensitivity, vision issues phototherapy is considered second-line therapy [12]. Today’s medical arsenal for treating psoriatic lesions allows for different drug delivery approaches to be considered based on clinical needs. Designing novel skin drug delivery systems with the goal of increasing the epidermal, dermal, and/or transdermal permeability of already identified active ingredients is one such approach [13]. To maintain the large interfacial region, therapeutic molecules must be submicron in size and spherical in shape, improving particle / skin interactions [14,15]. The improvement of skin drug deposition with a targeting effect, particularly in the epidermis, where psoriasis physiopathology is predominant, demonstrates a synergistic effect in combination with drug release that is sustained.
Discussion and Conclusion
Psoriasis pathogenesis is complex and multifaceted, with varying physiology, distribution, and severity. Significant development in understanding the complicated etiology of psoriasis and promoting the evolution of more credible therapies. In recent years, new methods of delivering drugs have improved patients’ quality by reducing the side effects of traditional therapy. Despite the progression, more research is yet required in several areas. Although there is no proven cure for psoriasis, some therapies can reduce or eliminate symptoms. In contrast, researchers have yet to identify and investigate the pathogenic correlation between psoriasis and other auto-inflammatory disorders.
References
- Boehncke WH, Schon MP (2015) Psoriasis: Electrochemical Behaviour of Tin (II) Chloride as a Solid-State Ionic Conductor. Lancet 4: 169-177.
- Trojacka E, Zaleska M, Galus R (2015) Influence of exogenous and endogenous factors on the course of psoriasis. Pol Merkur Lekarski 38(225): 169-173.
- Lonnberg AS, Skov L, Duffy DL, Skytthe A, Kyvik KO, et al. (2016) Genetic factors explain variation in the age at onset of psoriasis: A population-based twin study. Acta Derm Venereol 96(1): 35-38.
- Gulbahar S (2016) A short summary of clinical types of psoriasis. North Clin Istanbul 3(1): 79-82.
- Schoen M, Boehncke W (2005) Medical progress. N Engl J Med 352(18): 1899-1912.
- Fernandes AR, Martins-Gomes C, Santini A, Silva AM, Souto EB (2018) Psoriasis vulgaris-Pathophysiology of the disease and its classical treatment versus new drug delivery systems. In: Design of Nanostructures for Versatile Therapeutic Applications.
- Steinman L (2007) A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med 13(2):139-145.
- Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE (2010) Molecular dissection of psoriasis: Integrating genetics and biology. J Invest Dermatol 130(5):1213-1226.
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M (2005) Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J Exp Med 202(1):135-143.
- Di Cesare A, Di Meglio P, Nestle FO (2009) The IL-23Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 129(6):1339-1350.
- Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG (2008) Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 158(3): 558-566.
- Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM (2010) Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol 62(1):114-135.
- Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, et al. (2005) Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis.
- Schaller M, Preidel H, Januschke E, Korting HC (1999) Light and electron microscopic findings in a model of human cutaneous candidosis based on reconstructed human epidermis following the topical application of different econazole formulations. J Drug Target 6(5): 361-372.
- Maia CS, Mehnert W, Schaller M, Korting HC, Gysler A (2002) Drug targeting by solid lipid nanoparticles for dermal use. J Drug Target 10(6):489-495.
- Ortonne JP (1996) Aetiology and pathogenesis of psoriasis. Br J Dermatology 135(49): 1-5.






























