Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB): A Therapeutic Target for Gastrointestinal Tract Autoimmune Diseases
Jeyaparthasarathy Narayanaperumal, Nandhitha Madhusudhan, Avin D’souza, Miriyala Amarnath, and Ganesh Gopal*
ITC Limited, ITC Life Sciences and Technology Centre, Bangalore, India
Submission: March 13, 2023; Published: April 11, 2023
*Corresponding author: Ganesh Gopal, ITC Limited, ITC Life Sciences and Technology Centre. Bangalore, India
How to cite this article: Jeyaparthasarathy Narayanaperumal, Nandhitha Madhusudhan, Avin D’souza, Miriyala Amarnath, and Ganesh Gopal*. Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB): A Therapeutic Target for Gastrointestinal Tract Autoimmune Diseases. J of Pharmacol & Clin Res. 2023; 9(2): 555759. DOI: 10.19080/JPCR.2023.09.555759
Abstract
The immune system protects the body against diseases caused by bacteria, virus and fungi. As essential controllers of both innate and adaptive immune responses, activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway has a role in the development of immunological diseases. NF-κB is a well-known proinflammatory mediator and its deregulated activation has been linked to autoimmune disorders and chronic inflammation. Autoimmune disorders can arise when the immune system mistakenly attack healthy cells. An adaptive immune response specifically directed against a self-antigen is known as an autoimmune disease. Autoimmune diseases are broadly divided into two categories namely organ-specific and non-organ-specific (systemic). Some of the known organ-specific autoimmune diseases are multiple sclerosis (brain), autoimmune pancreatitis (pancreas), autoimmune hepatitis (liver), ulcerative colitis (gastrointestinal tract) and Hashimoto thyroiditis (thyroid). Therapeutic approaches are available for the treatment of autoimmune diseases, which are not completely efficient and have unfavourable side effects. As an alternative approach, phytochemicals and herbs are effective in fighting off a variety of diseases, including autoimmune disorders. This review aims to provide information about the use of phytochemicals in treating autoimmune diseases focusing on NF-κB signaling pathway specific to gastrointestinal tract.
Keywords: Autoimmune diseases; Celiac disease; Ulcerative colitis; Crohn’s disease; Inflammation; Immune response; NF-κB; Phytochemicals and Therapeutic
Abbreviations: NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; IL: Interleukin; Th: T helper cell; IFN: Interferon; TCR: T cell receptor; BCR: B cell receptor; Ig: Immunoglobulin; TNFR: TNF receptor; IκB: Inhibitory kappa B; MHC: Major Histocompatibility complex; CD: Cluster of differentiation; AIRE: Autoimmune Regulator; IRAK4: Interleukin-1 receptor-associated kinase-4
Introduction
The complex immune system evolved with the primary purpose of defending hosts against infectious pathogens. However, there are two major ways in which this pleiotropic immune system can lead to pathology: autoimmune disorders and immune deficiency syndromes [1]. Previously thought to be uncommon, autoimmune diseases are now known to impact 3-5% of the population and their effects on mortality and morbidity are significant. There are nearly 100 different autoimmune diseases, some of which are organ-specific and others are indicative of various immunological dysfunctions affecting multiple organs or systemic autoimmune diseases [2,3]. In general, autoimmune diseases are characterized by the predominance of T helper 1 (Th1) cytokines like interleukin-2 (IL-2) and interferon- γ (IFN-γ), and the effector responses typically involve cell-mediated immune responses, such as killing by cytotoxic T cells through the release of cytokines or by IgG and IgM antibodies directed against cell-surface antigens, which result in Fc receptor-mediated killing. Elevated levels of Th2 cytokines including IL-4, IL-5 and IL-10, the extensive circulation of autoantibodies and immune complex deposition, opsonization with antibody and cell injury via complement-mediated lysis are all characteristics of systemic autoimmune diseases [4]. NF-κB promotes the differentiation of Th1 cells by regulating T cell receptor (TCR) signaling and functioning in innate immunity to mediate the generation of cytokines like IL-12 that promote Th1 development. Th17 cells are characterized by the secretion of IL-17, an inflammatory cytokine that recruits monocytes and neutrophils to the site of inflammation in response to invasion by pathogens or self-antigens [5].
NF-κB Signaling Pathways
The canonical and non-canonical routes are the two main signaling pathways involved in the activation of NF-κB. Despite having different signaling mechanisms, both are crucial for controlling immunological and inflammatory responses [6,7]. The canonical NF-κB pathway reacts to a variety of stimuli, such as ligands for various cytokine receptors, pattern-recognition receptors (PRRs), members of the TNF receptor (TNFR) superfamily, TCR, and B-cell receptor (BCR) [8]. The inducible degradation of inhibitory kappa Bα (IκBα), which is brought on by its site-specific phosphorylation by a multi-subunit IκB kinase (IKK) complex, is the main mechanism for canonical NF-κB activation [9,10]. Two catalytic subunits, IKKα and IKKβ, as well as a regulatory component known as NF-κB essential modulator (NEMO) or IKKγ make up IKK [11]. Many stimuli such as cytokines, growth factors, mitogens, microbial components and stressors can activate IKK [12]. When IKK is activated, it phosphorylates IκBα at two N-terminal serines, which causes IκBα to be degraded in the proteasome in an ubiquitin-dependent manner. This causes the nuclear translocation of canonical NF-κB members, primarily the p50/RelA and p50/c-Rel dimers efficiently [13,10,14]. The non-canonical NF-κB pathway selectively reacts to a particular group of stimuli, such as ligands of a subset of TNFR superfamily members like lymphotoxin-β receptor (LTβR), B-cell activating factor receptor (BAFFR), Cluster of differentiation 40 (CD40) and receptor activator of NF-kappaB (RANK) [15,16].
Moreover, the non-canonical NF-κB activation relies on processing of the NF-κB2 precursor protein, p100, rather than IκBα degradation [6,15]. NF-κB-inducing kinase (NIK), a key signaling molecule for this pathway, activates and functionally collaborates with IKK to drive p100 phosphorylation, which in turn triggers p100 ubiquitination and processing [17,18]. Degradation of the C-terminal IκB-like structure of p100 occurs during processing, leading to the production of mature NF-κB2 p52 and nuclear translocation of the non-canonical NF-κB complex p52/RelB [6,8,15]. The non-canonical NF-κB route appears to have evolved as an additional signaling axis that works in conjunction with the canonical NF-κB pathway to regulate particular activities of the adaptive immune system whereas conventional NF-κB is functionally involved in practically all aspects of immune responses [16]. Regulation of inflammatory reactions is a well-known role of NF-κB. NF-κB controls the activation, differentiation and effector function of inflammatory T cells in addition to controlling the expression of several pro-inflammatory genes in innate immune cells [19,20]. Current research reveals that NF-κB also controls how inflammasomes are activated [21]. Evidently, chronic inflammatory disorders are characterized by dysregulated NF-κB activation. Due to this, it is crucial for therapeutic approaches in the treatment of inflammatory illnesses including autoimmune diseases to have a better knowledge of the process underlying NF- κB and pro-inflammatory activation.
Target Genes of NF-κB Signaling Pathways
NF-κB activates a variety of gene expressions including those encoding immunoreceptors, chemokines (IL-8, MIP-1a, MCP1, RANTES and eotaxin), cytokines (IL-1, IL-2, IL-6, IL-12, TNF-α, LTa and GM-CSF), proteins involved in antigen presentation, cell adhesion molecules (ICAM, VCAM and E-selectin), stress response genes, acute phase proteins, regulators of apoptosis, growth factors, inducible effector enzymes (iNOS and COX-2) and virusencoded genes [22]. Most of these target genes contribute to the regulation of the innate immune response. NF-κB also regulates the gene expression that provide adaptive immunity, including costimulatory B7.1, major histocompatibility complex (MHC) proteins and cytokines like IL-12, IL-2 and IFN-β [23]. NF-κB contribute to the overall immune response through activating genes coding for cell proliferation and apoptosis regulators.
NF-κB Signaling Pathways in Autoimmune Diseases
Immune response to self-antigens cause autoimmune diseases which in turn leads to tissue damage and chronic inflammation [24]. Many autoimmune disorders including rheumatoid arthritis, systemic lupus erythematosus, type I diabetes, multiple sclerosis and inflammatory bowel disease have been linked to NF-κB pathogenesis [25]. NF-κB activation is transiently present during a healthy immune response, but it is persistently active in the tissues of autoimmune disorders. Induction of proinflammatory cytokines and chemokines which facilitate the attraction of immune cells and formation of inflammation, is a well-known activity of NF-κB. Moreover, NF-κB influences the activity of dendritic cells to either directly or indirectly increase the activation of autoimmune T cells [26].
Autoimmune-Related NF-kB Signaling Pathway Inducers
Proinflammatory signals (cytokine receptors like TNFR family and IL-1R), toll-like receptors (TLRs) and the activation of lymphocyte receptors activate the canonical NF-κB pathway. When TLRs specific ligand is activated, it recruits MyD88 to activate the transcription factors NF-κB and interferon regulatory factors (IRF). As a result, the genes that code for different cytokines and type I interferons are activated. Since they produce proinflammatory cytokines, TLRs are crucial for the development of adaptive immunity. Its ongoing activation contributes to the pathophysiology of autoimmune disorders because of their function in innate and adaptive immunity. Defective clearance of apoptotic cell debris is another element in the pathophysiology of autoimmune disorders [27]. Systemic lupus erythematosus is largely brought on by the accumulation of nucleic acids in the cytoplasm as a result of inefficient clearance, which triggers endosomal TLR7 and TLR9. These have been demonstrated to be critical in the development of autoantibodies in a number of mice model studies [28]. MyD88 is necessary for their signaling as it binds to interleukin-1 receptor-associated kinase-4 (IRAK4) and causes IRAK1 and IRAK2 to become active. Hence, TNF receptorassociated factor 6 (TRAF6) is a target of IRAK1 and IRAK2. The transcription factors TRAF6, IRF7 and NF-κB are activated by K63-linked poly-ubiquitination [29]. There are ten members of the IL-1R family of receptors. Due to alternate splicing and proteolytic cleavages, each of these receptors has several variants. The receptors share internal TIR domains (Toll interleukin-1 receptor homology region) with TLR receptors and have three Iglike domains (D1, D2 and D3). The combinations of association between the various chains have led to the distinction of five different types of complexes which significantly activate NF-κB. These dysregulations are also linked to autoimmune or autoinflammatory disorders. IL-18, which is linked to autoimmune conditions such as multiple sclerosis, myasthenia gravis, rheumatoid arthritis, psoriasis, Becet’s syndrome, autoimmune thyroiditis, Crohn’s disease and type II diabetes activates the IL- 18R complexes [30].
Nucleotide-binding Oligomerization Domain-containing protein 1 and 2 (NOD1 and NOD2) are crucial intracellular pattern recognition receptors (PRRs) required to detect and regulate intracellular bacteria. NOD1 and NOD2 stimulate NF-κB, mitogenactivated protein kinases (MAPKs), autophagy and inflammasome to initiate immunological responses [31]. Even in the absence of stimulation by muramyl dipeptide and other bacterial wall cues, mutant NOD2 causes NF-κB hyper-activation [32]. Since NOD2 is less effective in identifying bacterial particles as a result of these hereditary mutations, germs can multiply and enter the intestinal mucosa [33]. These result in improper NF-κB activation and altered intestinal bacterial clearance [34,35]. The TNF family of cytokines which include Glucocorticoid-Induced Tumor Necrosis Factor (GITR), OX40, Herpes Virus Entry Mediator (HVEM), Death Receptor 3 (DR3), inducible costimulatory receptor (4- 1BB), CD30 and TNFR2 are primary targets for the therapy of autoimmune disorders due to their function as agonists in the immune response. The fact that all TNFR family receptors trigger NF-κB is one of their distinguishing characteristics. Autoimmune disorders are associated with high levels of TNF-α and TNFR. A class III TNF-α polymorphism is also linked to a large number of autoimmune disorders including Sjögren’s syndrome [36], systemic lupus erythematosus [37], rheumatoid arthritis [38] and ulcerative colitis [39]. DR3 is most abundantly expressed in antigen-presenting cells (APCs), phagocytes and activated T cells. It is also highly expressed in Crohn’s disease, ulcerative colitis and rheumatic diseases [40].
Autoimmune Diseases in Gastrointestinal tract
Chronic inflammatory disorders of the gastrointestinal tract are serious public health concern around the world. These conditions include Celiac disease and inflammatory bowel disorders (IBD) which includes Crohn’s disease and ulcerative colitis (UC) [41].
Celiac Disease
In Celiac Disease, a number of factors are responsible which include cellular susceptibility, pro-inflammatory properties of gluten and other wheat proteins, western diet and other environmental triggers which include viruses that increase the T cell-mediated response to gluten. The pro-inflammatory environment such as diet, viruses and cellular alterations that by themselves induce and/or make the cells more susceptible to pro-inflammatory factors are some of the causes that lead to proliferation of gliadin-specific T cells in genetically susceptible individuals and further shift them towards a pro-inflammatory phenotype [42]. Several genetic and expression studies have suggested that celiac disease alters the NF-κB pathway. It is widely recognized that NF-κB plays a crucial role in controlling the inducible gene expression in immune system. Moreover, it has been demonstrated that NF-κB mediates IL-15, a crucial component of innate immunity in celiac disease [43-46]. In comparison to control biopsies, a group of uncultured active and treated celiac disease patients, as well as cultured celiac disease biopsies at various stages of the disease (gluten-free diet; GFD, and gluten-containing diet; GCD) challenged with gliadin, showed altered expression of 93 genes linked to NF-κB [46]. These findings demonstrated that the genes that were constitutively upregulated in GFD-celiac disease patients belonged to the critical core of the pathway and had central and regulatory roles in the NF-κB signaling system. In comparison, genes that were overexpressed only in active celiac disease and appeared to be more peripheral and primarily comprised of NF-κB-inducible interleukins, adhesion molecules and receptors. Furthermore, the NF-κB pathway was upregulated by the gluten challenge in GFDceliac disease patient biopsies [47-49].
Ulcerative colitis
Ulcerative colitis (UC) is characterised by chronic and persistent inflammation of the intestinal mucosa. The course of the disease is influenced by a number of genetic factors, particularly polymorphisms in the interleukin, interleukin receptor and other inflammation-related genes [50]. The interleukin-1 gene family, the MDR1 multidrug resistance gene, the major histocompatibility complex (MHC) alleles HLA (human leukocyte antigen) class II and others have been identified as genetic susceptibility factors for the development of the disease [51]. The initial genetic link between colorectal cancer and UC is E-cadherin and genes involved in mucosal barrier function (ECM1, CDH1, HNF4, and laminin B1) are linked to an increased risk of UC [52]. The imbalance between excessive secretion of pro-inflammatory cytokines and relative insufficient secretion of anti-inflammatory cytokines is linked to the development of non-specific inflammatory responses in the intestine [53]. It indicates that NF-κB p65 highly expresses in intestinal mucosal epithelium, crypt epithelial cells and lamina propria monocytes of patients with UC and the expression of NF- κB in the nucleus is significantly higher than that in cytoplasm [54] NF-κB p65 antisense oligonucleotides blocks NF-κB pathway and down-regulates NF-κB-dependent IL-1beta and IL-8 mRNA expressions, which attenuates the productions of proinflammatory cytokines in lamina propria mononuclear cells from patients with UC [55].
Crohn’s disease
A complex condition of unclear aetiology, polygenic and environmental variables are major contributors to Crohn’s disease. Loss of tolerance to commensal flora antigens and aberrant activation of cellular immunity in the intestine are the implications [56]. An autoimmune response based on genetic and epigenetic factors, Crohn’s disease is a chronic disease of the small intestine caused by sensitivity to gluten and an immune system that refuses wheat proteins. This condition is genetically caused by a number of genes including HLA DQ, CTLA4, IL2 and others. Histone alterations and miRNA activity are two additional epigenetic mechanisms that are also at play. Current research on twins and Crohn’s disease affected families has demonstrated that some genetic profiles are susceptible to being affected [57]. HLA genes and non-HLA genes are the two groups of genes that are implicated in the pathophysiology of Crohn’s disease. They are crucial for the production of immune system mediators, cell signaling and other tissue damage mechanisms in the digestive tract [58]. In addition, the onset and progression of a disease are linked to abnormal responses to intestinal microbes and deregulation of NF-κB signaling pathways [59,60]. Chronic intestinal inflammation is caused by an excessive proinflammatory cytokine production that results from abnormal NF- κB activation. According to a recent study, patients with IBD had considerably higher NF-κB expression levels than those without IBD [61,62]. Moreover, immunostaining in the inflamed area compared to the non-inflamed areas in the same Crohn’s disease patients revealed a statistically significant increase in the number of NF-κB positive cells [62]. Current treatments are targeting NF- κB pathway, as it is crucial to the pathogenesis of IBD. Several studies have demonstrated that NF-κB activity declines with administration of anti-IBD drugs including corticosteroids and 5-aminosalycilic acid [63-65].
Currently, medications and surgery are the hallmarks of therapeutic care for autoimmune diseases of the gastrointestinal tract. In order to reduce inflammation and promote mucosal healing, the current pharmacological therapies include 5-aminosalicylate, substances like sulfasalazine, mesalamine, corticosteroids (cortisone and budesonide), immunomodulators (thiopurines and methotrexate), anti-TNF agents (infliximab, adalimumab, golimumab), anti-integrins (Vedolizumab) and calcineurin inhibitors (cyclosporine) [66]. However, long-term use of these medications can result in serious consequences and unfavourable side effects, such as gastrointestinal problems, systemic immunosuppression, renal toxicity, diabetes, weight gain, high blood pressure and rise in infections [67,68]. In vitro and in vivo studies have demonstrated the effectiveness of many natural compounds such as flavonoids, terpenoids and phenolic acids in treating various inflammatory autoimmune disorders without causing any adverse effects [69,70]. According to research, phytochemicals in fruits, vegetables and herbs can inhibit enzymes, down-regulate the immune response and suppress the production of pro-inflammatory cytokines which helps to prevent the development of autoimmune diseases. Plants produce a variety of chemical compounds known as phytochemicals through primary and secondary metabolism. Around 12,000 phytochemicals have been discovered by scientific research [71]. Phytochemicals are divided into polyphenols, alkaloids, terpenoids, lignans, saponins and organ sulfides based on their origin, chemical structure and functions. They have a wide range of potential health advantages including antibacterial, antioxidant activities, immune system stimulation and detoxifying enzyme modulation [72]. The following list includes some phytochemicals that modulate the NF-κB signaling pathway (Table 1).
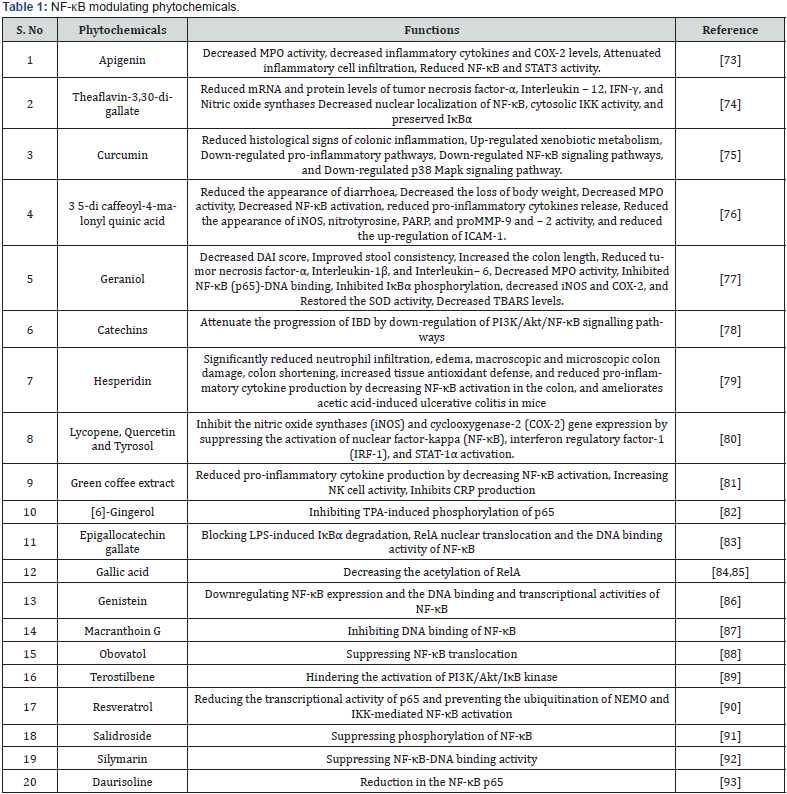
Discussion and Conclusion
It is now widely accepted that NF-κB acts as a key mediator of inflammation that triggers wide range of immune receptors. Targeting the NF-κB signaling pathway for anti-inflammatory therapeutics is of prime importance since uncontrolled NF- κB activation contributes to a number of gastrointestinal tract autoimmune disorders such as Celiac disease, Crohn’s disease and Ulcerative colitis. Several types of inhibitors have been developed to block various NF-κB signaling pathways which may be of therapeutic importance in treating gastrointestinal tract autoimmune diseases. Some of them are
a) Excessive activation of TLRs disturb immune homeostasis and as a result, promotes the onset of numerous inflammatory and autoimmune diseases related to gastrointestinal tract [94]. Therefore, TLR signal-targeting inhibitors and antagonists may be useful for the treatment of these diseases.
b) MyD88 is a vital adapter protein involved in the signaling of the IL-1 and Toll-like receptor families, which regulates innate immune responses and inflammation [95]. With a focus on gastrointestinal tract autoimmune diseases, the development of MyD88 dimerization disruptors provides an innovative therapeutic approach.
c) Targeted IKK inhibitors are being developed to inhibit catalytic activity of IKK and prevent IκBα phosphorylation [96]. IKK can also be inhibited by some well-known anti-inflammatory drugs, including aspirin and salicylate [97].
d) Proteasome inhibitors like lactacystin and Velcade (also known as Bortezomib and PS-341) prevent IκBα breakdown in the proteasome.
e) NF-κB subunit inhibitors that prevent nuclear translocation include tacrolimus (FK-506) and the IκBα superrepressor.
f) Drugs like glucocorticoids and peroxisome proliferatoractivated receptors agonists that block DNA-binding activity of NF-κB.
While NF-κB inhibitors have advanced significantly, it is still difficult to develop NF-κB-based drugs that are clinically effective. Although NF-κB suppression may be helpful in the treatment of inflammatory diseases, as its function is also necessary for preserving healthy immune responses and cell survival, there are apparent concerns about the balance between efficacy and safety. Increasing evidence suggests that serious side effects could result from the suppression of NF-κB signaling. Therefore, developing more specific and potent therapeutic formulations from phytochemicals (Figure 1) for the treatment of autoimmune disorders including gastrointestinal tract related autoimmune diseases requires a better understanding of the mechanism behind the pathogenic activation of NF-κB.
Acknowledgements
We acknowledge our chief scientist Dr. Suresh Ramamurthi for his encouragement/support for the manuscript.
- Review Article
- Abstract
- Introduction
- Autoimmune Diseases in Gastrointestinal tract
- Discussion and Conclusion
- Acknowledgements
- References
References
- Wang L, Wang FS, Gershwin ME (2015) Human autoimmune diseases: A comprehensive update. J Intern Med 278(4): 369-395.
- Jacobson DL, Gange SJ, Rose NR, Graham NM (1997) Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84(3): 223-243.
- Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB (2007) Epidemiology of autoimmune diseases in Denmark. J Autoimmun 29(1): 1-9.
- Smith DA, Germolec DR (1999) Introduction to immunology and autoimmunity. Environ Health Perspect 107(Suppl 5): 661-665.
- Oh H, Ghosh S (2013) NF-kappaB: Roles and regulation in different CD4(+) T-cell subsets. Immunol Rev 252(1): 41-51.
- Sun SC (2011) Noncanonical NF-kappaB signaling pathway. Cell Res 21(1): 71-85.
- Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27: 693-733.
- Zhang H, Sun SC (2015) NF-kappaB in inflammation and renal diseases. Cell Biosci 5: 63.
- Oeckinghaus A, Ghosh S (2009) The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor Perspect Biol 1(4): a000034.
- Karin M, Delhase M (2000) The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signaling. Semi Immunol 12(1): 85-98.
- Sun SC, Ley SC (2008) New insights into NF-kappaB regulation and function. Trends Immunol 29(10): 469-478.
- Israël A (2010) The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harbor Perspect Biol 2(3): a000158.
- Beinke S, Ley SC (2004) Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J 382(2): 393-409.
- Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132(3): 344-362.
- Sun SC (2012) The noncanonical NF-kappaB pathway. Immunol Rev 246(1): 125-140.
- Sun SC, Liu ZG (2011) A special issue on NF-kappaB signaling and function. Cell Res 21(1): 1-2.
- Xiao G, Harhaj EW, Sun SC (2001) NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell 7(2): 401-409.
- Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, et al. (2001) Activation by IKK alpha of a second, evolutionary conserved, NF-kappaB signaling pathway. Science 293(5534): 1495-1499.
- Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspect Biol 1(6): a001651.
- Tak PP, Firestein GS (2001) NF-kappaB: A key role in inflammatory diseases. J Clin Invest 107(1): 7-11.
- Sutterwala FS, Haasken S, Cassel SL (2014) Mechanism of NLRP3 inflammasome activation Ann N Y Acad Sci 1319(1): 82-95.
- Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109: S81-S96.
- Hoffmann A, Levchenko A, Scott ML, Baltimore D (2002) The Kappa-NF-kappaB signaling module: Temporal control and selective gene activation. Science 298(5596): 1241-1245.
- Theofilopoulos AN (1995) The basis of autoimmunity: Part I. Mechanisms of aberrant self-recognition. Immunol Today 16(2): 90-98.
- Pai S, Thomas R (2008) Immune deficiency or hyperactivity-NF-kappaB illuminates autoimmunity. J Autoimmun 31(3): 245-251.
- Sun SC, Chang JH, Jin J (2013) Regulation of nuclear factor-κB in autoimmunity. Trends Immunol 34(6): 282-289.
- Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M (2010) The role of defective clearance of apoptotic cells in systemic autoimmunity. Rheumatology 6(5): 280-289.
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, et al. (2011) Linear ubiquitination prevents inflammation and regulates immune signaling. Nature 471(7340), 591-596.
- Suthers AN (2017) Sarantopoulos SJFii. TLR7/TLR9-and B cell Receptor Signaling crosstalk: Promotion of potentially dangerous B cells. Front Immunol 8: 775.
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA (1999) Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity 10(1): 127-136.
- Keestra-Gounder AM, Tsolis RM (2017) NOD1 and NOD2: Beyond peptidoglycan sensing. Trends Immunol 38(10): 758-767.
- Dziadzio M, Ammann S, Canning C, Boyle F, Hassan A, et al. (2015) Symptomatic males and female carriers in a Large Caucasian kindred with XIAP deficiency. J Clin Immunol 35(5): 439-444.
- Eckmann L, Karin MJI (2005) NOD2 and Crohn’s disease: Loss or gain of function? Immunity 22(6): 661-667.
- Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, et al. (2004) NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal a-defensin expression. Gut 53(11): 1658-1664.
- Tan G, Liang E, Liao K, Deng F, Zhang W, et al. (2016) NOD2 Upregulates TLR2-mediated IL-23p19 expression via NF-kB subunit C-Rel in Paneth cell-like cells. Oncotarget 7(39): 63651-63660.
- Guggenbuhl P, Veillard E, Quelvenec E, Jego P, Semana G, et al. (2000) Analysis of TNFalpha microsatellites in 35 patients with primary Sjögren’s syndrome. Joint Bone Spine 67(4): 290-295.
- Rood MJ, Van Krugten MV, Zanelli E, Vander Linden MW, Keijsers V, et al. (2000) TNF-308A and HLA-DR3 alleles contribute independently to susceptibility to systemic lupus erythematosus. Arthritis Rheum 43(1): 129-134.
- Werth VP, Zhang W, Dortzbach K, Sullivan K (2000) Association of a promoter polymorphism of tumor necrosis factor-a with subacute cutaneous lupus erythematosus and distinct photo regulation of transcription. J Invest Dermatol 115(4): 726-730.
- Martinez A, Fernendez-Arquero M, Pascual-Salcedo D, Conejero L, Alves H, et al. (2000) Primary Association of Tumor Necrosis Factor-Region Genetic Markers With Susceptibility to Rheumatoid Arthritis. Arthritis Rheum 43(6): 1366-1370.
- Aiba Y, Nakamura M (2013) The role of TL1A and DR3 in autoimmune and inflammatory diseases. Mediators Inflamm pp: 258164.
- Ghouri YA, Tahan V, Shen B (2020) Secondary causes of inflammatory bowel diseases. World J Gastroenterol 26(28): 3998-4017.
- Barone MV, Auricchio R, Nanayakkara M, Greco L, Troncone R (2022) Pivotal role of inflammation in celiac disease. Int J Mol Sci 23(13): 7177.
- Trynka G, Zhernakova A, Romanos J, Franke L, Hunt KA, et al. (2009) Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-kappaB signaling. Gut 58(8): 1078-1083.
- Fernandez-Jimenez N, Castellanos-Rubio A, Plaza-Izurieta L, Irastorza I, Elcoroaristizabal X, et al. (2014) Coregulation and modulation of NFκB-related genes in celiac disease: Uncovered aspects of gut mucosal inflammation. Hum Mol Genet 23(5): 1298-1310.
- Castellanos-Rubio A, Bilbao JR (2018) Profiling celiac disease-related transcriptional changes. Int Rev Cell Mol Biol 336: 149-174.
- Castellanos-Rubio A, Ghosh S (2019) Disease-associated SNPs in inflammation-related lncRNAs. Front Immunol 10: 420.
- Fernandez-Jimenez N, Castellanos-Rubio A, Plaza-Izurieta L, Irastorza I, Elcoroaristizabal X, et al. (2014) Coregulation and modulation of NFκB-related genes in celiac disease: Uncovered aspects of gut mucosal inflammation. Hum Mol Genet 23(5): 1298-1310.
- Castellanos-Rubio A, Bilbao JR (2018) Profiling celiac disease-related transcriptional changes. Int Rev Cell Mol Biol 336: 149-174.
- Castellanos-Rubio A, Ghosh S (2019) Disease-associated SNPs in inflammation-related lncRNAs. Front Immunol 10: 420.
- Sarlos P, Kovesdi E, Magyari L, Banfai Z, Szabo A (2014) Genetic update on inflammatory factors in ulcerative colitis: Review of the current literature. World J Gastrointest Pathophysiol 5(3): 304-321.
- Thompson AI, Lees CW (2011) Genetics of ulcerative colitis. Inflamm Bowel Dis 17(3): 831-848.
- Ahmad T, Armuzzi A, Neville M, Bunce M, Ling KL, et al. (2003) The contribution of human leucocyte antigen complex genes to disease phenotype in ulcerative colitis. Tissue Antigens 62(6): 527-535.
- Woodford-Richens K, Bevan S, Churchman M, Dowling B, Jones D, et al. (2000) Analysis of genetic and phenotypic heterogeneity in juvenile polyposis. Gut 46(5): 656-660.
- Yu ZH, Huang F, Xu N, Zhao DM, Hu FA, et al. (2011) Expression of toll-like receptor 4, CD14, and NF-κB in Chinese patients with ulcerative colitis. J Immunoassay Immunochem 32(1): 47-56.
- Li Z, Zhang DK, Yi WQ, Ouyang Q, Chen YQ (2008) NF-κB p65 antisense oligonucleotides may serve as a novel molecular approach for the treatment of patients with ulcerative colitis. Arch Med Res 39(8): 729-734.
- Lamoril J, Deybach JC, Bouizegarène P (2007) Maladie de Crohn et génétique: Connaissances actuelles. Immuno-Analyse and Biologie Spécialisée 22(3): 137-150.
- Louka AS, Sollid LM (2003) HLA in coeliac disease: Unravelling the complex genetics of a complex disorder. Tissue Antigens 61(2): 105-117.
- Falchuk ZM, Rogentine GN, Strober W (1972) Predominance of histocompatibility antigen HL-A8 in patients with gluten-sensitive enteropathy. J Clin Invest 51(6): 1602-1605.
- Vora P, Shih DQ, McGovern DP, Targan SR (2012) Current concepts on the immunopathogenesis of inflammatory bowel disease. Front Biosci 4(4): 1451-1477.
- Shih DQ, Targan SR (2008) Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol 14(3): 390-400.
- Zhang C, Liu LW, Sun WJ, Qin SH, Qin LZ (2015) Expressions of E-cadherin, p120ctn, beta-catenin and NF-kappaB in ulcerative colitis. J Huazhong Univ Sci Technolog Med Sci 35(3): 368-373.
- Ellis RD, Goodlad JR, Limb GA, Powell JJ, Thompson RP (1998) Activation of nuclear factor kappa B in Crohn’s disease. Inflamm Res 47(11): 440-445.
- Schreiber S, Nikolaus S, Hampe J (1998) Activation of nuclear factor kappa B inflammatory bowel disease. Gut 42(4): 477-484.
- Gan HT, Chen YQ, Ouyang Q (2005) Sulfasalazine inhibits activation of nuclear factor-kappaB in patients with ulcerative colitis. J Gastroenterol Hepatol 20(7): 1016-1024.
- Bantel H, Berg C, Vieth M, Stolte M, Kruis W (2000) Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol 95(12): 3452-3457.
- Tripathi K, Feuerstein JD (2019) New developments in ulcerative colitis: Latest evidence on management, treatment, and maintenance. Drugs Context 8: 212572.
- Hagan M, Hayee BH, Rodriguez-Mateos A (2021) (Poly)phenols in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Review. Molecules 26(7): 1843.
- Shen C, Zhao L, Du X, Tian J, Yuan Y (2021) Smart responsive quercetin-conjugated glycol chitosan prodrug micelles for treatment of inflammatory bowel diseases. Mol Pharm 18(3): 1419-1430.
- Dönder Y, Arikan TB, Baykan M, Akyüz M, Öz AB (2018) Effects of quercitrin on bacterial translocation in a rat model of experimental colitis. Asian J Surg 41(6): 543-550.
- Qin HY, Zang KH, Zuo X, Wu XA, Bian ZX (2019) Quercetin attenuates visceral hypersensitivity and 5-hydroxytryptamine availability in post inflammatory irritable bowel syndrome rats: Role of enterochromaffin cells in the colon. J Med Food 22(7): 663-671.
- Hossen I, Hua W, Ting L, Mehmood A, Jingyi S, et al. (2022) Phytochemicals and inflammatory bowel disease: A review. Crit Rev Food Sci Nutr 60: 1321-1345.
- Campos-Vega R, Oomah BD (2013) Chemistry and classification of phytochemicals. Handb. Plant Food Phytochem 5-48.
- Jia H, Zhang Y, Si X, Jin Y, Jiang D (2021) Quercetin alleviates oxidative damage by activating nuclear factor erythroid 2-related factor 2 signaling in porcine enterocytes. Nutrients 13(2): 375.
- Ukil A, Maity S, Das PK (2006) Protection from experimental colitis by theaflavin-3,3′-digallate correlates with inhibition of IKK and NF− κ B activation: Protection of colitis by TFDG via NF− κ B pathway. B Journal of Pharmacology 149(1): 121-131.
- Nones K, Dommels YEM, Martell S, Butts C, McNabb WC, et al. (2009) The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a −/−) mice, a model of inflammatory bowel diseases. B J Nutr 101(2): 169-181.
- Di paola R, Esposito E, Mazzon E, Caminiti R, Toso RD (2010) 3,5-Dicaffeoyl-4-malonylquinic acid reduced oxidative stress and inflammation in a experimental model of inflammatory bowel disease. Free Radic Res 44(1): 74-89.
- Medicherla K, Sahu BD, Kuncha M, Kumar JM, Sudhakar G (2015) Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct 6(9): 2984-2995.
- Chiou YS, Ma NJL, Sang S, Ho CT, Wang YJ (2012) Peracetylated (-)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J Agri Food Chem 60(13): 3441-3451.
- Guazelli CFS, Fattori V, Ferraz CR, Borghi SM, Casagrande R, Baracat, et al. (2021) Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem Biol Interact 333:109315.
- De Stefano D, Maiuri MC, Simeon V, Grassia G, Soscia A (2007) Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur J Pharmacol 566(1-3): 192-199.
- Narayanaperumal J, D’souza A, Miriyala A, Sharma B, Gopal G (2022). A randomized double blinded placebo controlled clinical trial for the evaluation of green coffee extract on immune health in healthy adults. J Tradit Complement Med 12(5): 455-465.
- Kim SO, Kundu JK, Shin YK, Park JH, Cho MH (2005) Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-κB in phorbol ester-stimulated mouse skin. Oncogene 24(15): 2558-2567.
- Joo SY, Song YA, Park YL, Myung E, Chung CY, et al. (2012) Epigallocatechin-3-gallate inhibits LPS-induced NF-κB and MAPK signaling pathways in bone marrow-derived macrophages. Gut Liver 6(2): 188-196.
- Choi KC, Lee YH, Jung MG, Kwon SH, Kim M, et al. (2009) Gallic acid suppresses lipopolysaccharide- induced nuclear factor-κB signaling by preventing RelA acetylation in A549 lung cancer cells. Mol Cancer Res 7(12): 2011-2021.
- Kim MJ, Seong AR, Yoo JY, Jin CH, Lee YH, et al. (2011) Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol Nutr Food Res 55(12): 1798-1808.
- Zhou X, Yuan L, Zhao X, Hou C, Ma W (2014) Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-κ Nutrition 30(1): 90-95.
- Hu WC, Wang GC, Li PX, Wang Y, Si CL, et al. (2014) Neuroprotective effects of macranthoin G from Eucommia ulmoides against hydrogen peroxide-induced apoptosis in PC12 cells via inhibiting NF-κB activation. Chem Biol Interact 224: 108-116.
- Choi MS, Lee SH, Cho HS, Kim Y, Yun YP, et al. (2007) Inhibitory effect of obovatol on nitric oxide production and activation of NF-κB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. European Journal of Pharmacology 556(1-3): 181-189.
- Wang YJ, Elsevier CRJ (2014) Pterostilbene Protection and Bladder Cancer Cells. Cancer pp: 271-281.
- Ren ZH, Wang L, Cui JH, Huoc Z, Xue J (2013) Resveratrol inhibits NF-κB signaling through suppression of p65 and IκB kinase activities. Pharmazie 68(8): 689-694.
- Wang JW, Pan YB, Cao YQ, Zhou W, Lu J (2019) Salidroside regulates the expressions of IL-6 and defensins in LPS-activated intestinal epithelial cells through NF-κB/MAPK and STAT3 pathways. Iran J Basic Med Sci 22(1): 31-37.
- Saliou C, Rihn B, Cillard J, Okamoto T, Packer L (1998) Selective inhibition of NF-κB activation by the flavonoid hepatoprotector silymarin in Hep G2. Evidence for different activating pathways. FEBS Letters 440(1-2): 8-12.
- Zhou J, Wu H, Hou J, Wang J, Wang J, et al. (2022) Daurisoline alleviated experimental colitis in vivo and in vitro: Involvement of NF-kB and Wnt/-Catenin pathway. Int Immunopharmacology 108: 108714.
- Gao W, Xiong Y, Li Q, Yang H (2017) Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: A journey from molecular to Nano therapeutics. Front Physiol 8: 508.
- Di Padova F, Quesniaux VFJ, Ryffel B (2018) MyD88 as a therapeutic target for inflammatory lung diseases. Expert Opin Ther Targets 22(5): 401-408.
- Lin Y, Bai L, Chen W, Xu S (2010) The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets 14(1): 45-55.
- Yin MJ, Yamamoto Y, Gaynor RB (1998) The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396(6706): 77-80.






























