Effect of Albendazole on the kinetics of Sulphadimidine in West African Dwarf (WAD) Goats following Intramuscular Administration
Agbo JO*, Bosha JA, Saganuwan SA and Onyeyili PA
Department of Veterinary Pharmacology and Toxicology, Federal University of Agriculture Makurdi, Benue State, Nigeria
Submission: December 19, 2022; Published: January 03, 2023
*Corresponding author:Agbo JO, Department of Veterinary Pharmacology and Toxicology, Federal University of Agriculture Makurdi, Benue State, Nigeria
How to cite this article:Agbo JO, Bosha JA, Saganuwan SA, Onyeyili PA . Effect of Albendazole on the kinetics of Sulphadimidine in West African Dwarf (WAD) Goats following Intramuscular Administration. J of Pharmacol & Clin Res. 2023; 9(1): 555752. DOI: 10.19080/JPCR.2023.09.555752
Abstract
Cases of mixed infections requiring the use of antimicrobials and anthelminthics are common in veterinary medicine. In view of this, the effect of albendazole on the kinetics of sulphadimidine was studied in eight WAD goats of 1-2 years old with 12.44 ± 0.83 kg mean body weight separated into two groups of four animals each. Sulphadimidine sodium (33.3%) and albendazole (2.5%) were administered intramuscularly at a dose of 100 and 15 mg/kg body weight, respectively. The elimination half-life (5.86±0.59 h) was significantly lower (p<0.05) in the sulphadimidine treated group as compared to the half-life of sulphadimidine/albendazole treated group respectively. However, volume of distribution (Vd) (9.76±1.03 L/kg) and total body clearance (Cl) (1.21±0.22 L/kg/h) were significantly higher (p<0.05) in sulphadimidine treated group as compared to Vd (7.31±0.80 L/kg) and Cl (0.67±0.06 L/kg/h) of the sulphadimidine/albendazole treated group, respectively. Hence albendazole could increase elimination half-life of sulphadimidine, invariably increasing the duration of sulphadimidine antimicrobial activity.
Keywords: Pharmacokinetic; Sulphadimidine; African Dwarf goat; Albendazole; Half-life
Abbreviations: TCA: Trichloroacetic Acid; WAD: West African Dwarf; ICLAS: International Council for Laboratory Animal Science; LOD: Limit of Detection; LOQ: Limit of Quantification
Introduction
Albendazole is extensively used in the treatment of gastrointestinal helminthosis. It has activity against liver flukes, lungworm and migrating larvae with low levels of toxicity in animals [1]. Albendazole inhibit the assembly of microtubule in the sensitive nematode, whereas its metabolite albendazole sulfoxide is responsible for anthelmintic activity [2,3]. Sulphadimidine, a synthetic antimicrobial drug is widely used in the treatment of animal diseases caused by micro-organisms including norcardia, actinomyces, toxoplasma and coccidiosis [4,5]. Low cost and effectiveness are responsible for its widespread use [6]. The kinetics of sulphadimidine has been studied in several species of animals including cattle, goat, sheep, broilers, turkey, guinea fowl, domestic chicken, duck, rabbits and dogs [7-12]. Sulphadimidine is used in combination with albendazole in the treatment of infections caused by microorganisms sensitive to sulphadimidine and when there is a concurrent diagnosis of helminthosis. Literature reviews have shown no information on the possible interaction between the combination of both drugs. In view of the above, the effect of albendazole on the pharmacokinetics of sulphadimidine was studied in West African Dwarf goats.
Materials
The materials used during the research are Trichloroacetic acid (TCA) manufactured by (Guangdong, China), sodium nitrite (Kermel, China), sulphamic acid (BDH Chemicals, England), 8-OH quinoline (Sinopharm, China), sodium hydroxide (Qualikems, India), distilled water, sulphadimidine sodium (Shijiazhuang Guanghua Pharma. Co, Ltd. China), albendazole (Shijiazhuang Guanghua Pharma. Co, Ltd. China) , uv spectrophotometer, vortex mixer, test tubes and test tube rack, measuring cylinder, weighing scale, spatula, lithium heparin tubes, needle and syringes (2 ml).
Animals
This study was carried out in the laboratory of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Federal University of Agriculture, Makurdi. The West African Dwarf (WAD) goats were purchased from local breeders in Makurdi metropolis. The goats were screened for the presence of endo and ectoparasites and stabilized for two weeks prior to experimentation. Concentrate and water were provided ad libitum. All the animals were handled according to the international guiding principle for biomedical research involving animals [International Council for Laboratory Animal Science [ICLAS] and Council for International Organization of Medical Sciences (CIOMS),[13] as permitted by the College of Veterinary Medicine, Federal University of Agriculture, Makurdi, Ethical Committee.
Experimental design and drug administration
Eight (8) WAD goats of 1- 2 years old that weighed 12.44 ± 0.83 kg mean weight were separated into two groups of four animals, consisting of two males and female each. Sulphadimdine sodium (33.3%) and albendazole (2.5%) were administered at doses of 100 and 15 mg/kg body weight respectively. Sulphadimidine was administered on the right gluteal muscle to goats. The goats in group 2 also received albendazole suspension orally at the same time.
Collection blood sample
Blood sample (2.0 ml) was collected through the left jugular vein of each goat prior to sulphadimidine administration and at 15, 30, 45 min and 1, 2, 4, 6, 8, 10, 12, 24, 48 hr. The samples collected into heparinized tubes using 23 G disposable needle and 2 ml syringe were centrifuged at 3000 rpm for 10 min. The plasma was obtained and stored at -20°C until analysed.
Determination of sulphadimidine in plasma
Concentration of sulphadimidine in plasma was determined by a modified chemical assay method described by Nagaraja et al. [14]. The method is based on the diazotization of sulphadimidine and coupling with 8-OH quinoline in alkaline media to yield red colored products with absorption maxima at 500 nm. To 0.4 ml of plasma in a 5 ml glass test tube, 2 ml of distilled water was added, followed by 0.6 ml of 20% trichloroacetic acid. The resulting solution was mixed using a vortex mixer and centrifuged for 5 min. To the clear supernatant (2.5 ml) 0.15 ml of 1.0% sodium nitrite was added, mixed and allowed to stand for 5 min. Thereafter, 0.25 ml of 2.0% sulphamic acid was added, mixed and allowed to stand for 5 min. This was followed by addition of 0.2 ml of 0.5% 8-OH quinoline. The resulting solution was mixed and allowed to stand for 5 min, followed by addition of 0.2 ml of 20% sodium hydroxide. The resulting solution was mixed, and the absorbance read using a UV-spectrophotometer (spectrum lab 23A, 340- 1000 nm) at a wavelength of 500 nm. The linear calibration curve of sulphadimidine in plasma within the range of 1.25-20.0 μg/ml was obtained by plotting absorbance on y-axis against time on x-axis with linear regression showed R2=0.998. The limit of detection (LOD) and limit of quantification (LOQ) of sulphadimidine in plasma were 0.08 and 0.24 μg/ml respectively. The intra-day and inter-day precision were 0.50 and 0.80 % respectively.
Calculation of pharmacokinetic parameters
The pharmacokinetic parameters for individual animals were calculated using established kinetic equations [2,15,16] and pharmacokinetic software (Kinetica 5.0, Thermo Fischer Scientific). Micro constants (α, β) were determined from all the plotted graphs obtained from the generated data. Statistical analysis. The data on plasma kinetics and pharmacokinetic parameters were presented in graphical and tabular form respectively. Plasma concentrations and pharmacokinetic parameters were presented as Average ± Standard Error of Mean (SEM) and analyzed Graph Pad Prism 6.03 for windows at 5% level of significance.
Results
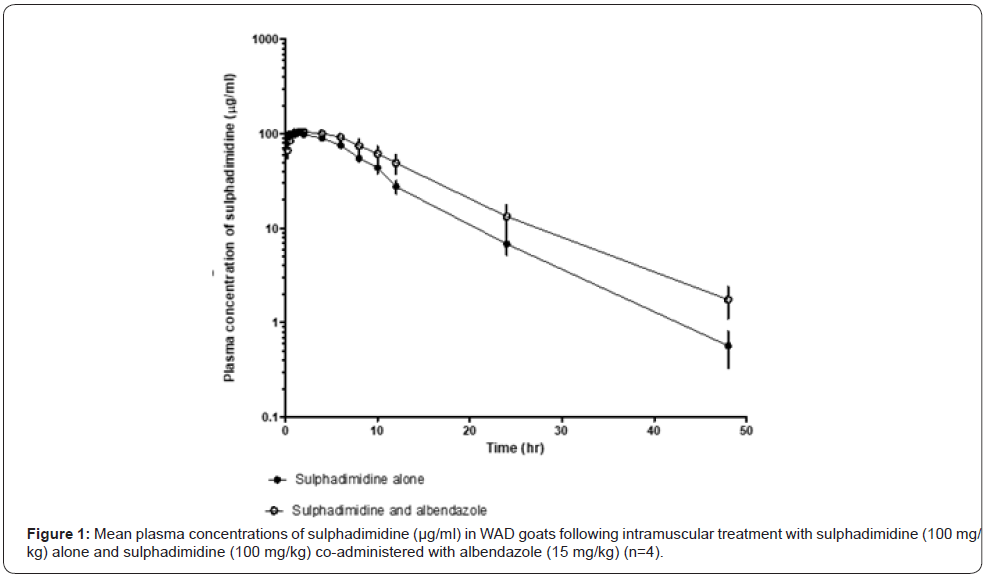
The pharmacokinetic parameters of sulphadimidine in WAD goats administered sulphadimidine alone and sulphadimidine with albendazole is shown in Table 1. Concentrations of sulphadimidine free amine were detected for a period of 48 h. The kinetic disposition of sulphadimidine indicated that the data best fitted a two compartmental open model (Figure 1). There was significant difference (p<0.05) between absorption rate constant α (0.24±0.05/h), absorption half-life (T1/2α) (3.10±0.57 h), mean absorption time (MAT) (4.98±0.44 h), elimination half-life (T1/2β) (5.86±0.59 h), volume of distribution (Vd) (9.76±1.03 L/ kg), total body clearance (Cl) (1.21±0.22 L/kg/h), mean residence time (MRT) (8.22±0.78 h), area under the curve from zero to 48 h (AUC0-48) (1132.77±91.71 μg/ml.h), area under the curve from zero to infinity (AUC0-∞) (1141.99±90.51 μg/ml.h) and area under the moment curve (AUMC) (8795.26±1666.89 μg/ml.h2) in the sulphadimidine treated group as compared to the α (0.76±0.08/h), T1/2α (0.93±0.10 h), MAT (1.34±0.14 h), T1/2β (7.42±0.41 h), Vd (7.31±0.80 L/kg), Cl (0.67±0.06 L/kg/h), MRT (11.52±0.30 h), AUC0-48 (1766.16 μg/ml.h), AUC0-∞ (1788.98 μg/ml.h), and AUMC (18322.59±1749.40) of the WAD goats administered sulphadimidine and albendazole treated group (Table 1).Data are presented as mean±SEM; a= significantly higher; b = significantly lower; p<0.05. Cmax= maximum concentration; Tmax= time of maximum concentration; α= absorption rate constant; T1/2α= absorption half-life; β= elimination rate constant; T1/2β= elimination half-life; Vd= volume of distribution; Cl= total body clearance; MRT= mean residence time; AUC0-t= area under the plasma concentration vs time curve from 0 to 48 h; AUC0-∞= area under the plasma concentration vs time curve from 0 to infinite; AUMC= Area under the moment curve.

Discussion
The pharmacokinetic parameters of sulphadimidine and sulphadimidine in combination with albendazole best fit a two-compartment open model (Figure 1). Our findings agree with the report of Akogwu et al. [7], indicating that intramuscular sulphadimidine obeys first order kinetics in WAD goats. Sulphadimidine has also been reported to follow a two-compartment open model in turkey poults, cows, dogs and buffaloes [10, 17-19]. However, our findings are at variance with the observation in guinea fowls and turkey poults where the disposition kinetics of sulphadimidine was described by monoexponential decline in plasma levels of the drug with passage of time after administration. The higher absorption rate constant observed in the sulphadimidine/albendazole group shows that albendazole can increase absorption of sulphadimidine. Our findings agree with the report of Saganwan [20] indicating that disposition kinetics of albendazole is dependent on the volume of distribution and could be antagonistic. Metabolites of albendazole could display different disposition kinetics [20] The elimination half-life of sulphadimidine in the WAD goats administered sulphadimidine and albendazole concurrently is higher than the reported values in sheep (4.72±0.26 h) [8] and Nubian goat (4.13 h) [21] and similar to the value reported in WAD goat (7.24±0.59 h) [7]. However, the elimination half-life of sulphadimidine with albendazole is lower than that of cattle (9.46 ±0.93 h, 8.32±0.96 h) [22] and sheep (9.51 h) [23]. This may be due to the differences in species, route of administration and analytical method employed. This also indicates that kinetics of sulphadimidine differ from one animal to another even among the same animal species [24].
The volume of distribution in animals administered sulphadimidine alone (9.76±1.03 L/kg) and those treated with the combination of sulphadimidine and albendazole (7.31±0.80 L/kg) showed that sulphadimidine has high volume of distribution in WAD goats. These volumes of distributions were higher than those of the dog (0.68±0.12 L/kg), sheep (0.6±0.11 L/kg), guinea fowl (1.29±0.47 L/kg), ducks (2.33±0.36 L/kg) and WAD goat (3.39±0.38 L/kg) [7,11,18,25]. Concurrent administration of sulphadimidine and albendzole decreased the volume of distribution and increased plasma concentrations of sulphadimidine as seen [26,27] (Figure 1). Total body clearance is known to reflect the elimination of drug from the body [20]. The higher the drug concentration in the plasma, the faster the drugs are eliminated as observed in the present study. Treatment with sulphadimidine alone decreased AUC of the drug as compared with that of sulphadimidine/albendazole, signifying that albendazole can decrease disposition kinetics of sulphadimidine.
Conclusion
Albendazole decreased absorption half-life, mean absorption time, volume of distribution and total clearance of sulphadimidine in WAD goats. On the contrary, albendazole increased absorption rate constant, elimination half-life, mean residence time, area under curve and area under moment curve of sulphadimidine. Hence administration of intramuscular sulphadimidine and albendazole should be done carefully to avoid crystalluria. Administration of albendazole in combination with sulphadimidine significantly altered the kinetic parameters of sulphadimidine in WAD goats. The combination increased the sulphadimidine concentrations in plasma above the minimal inhibitory concentration for several hours when compared to that of sulphadimidine treatment alone. This implies a synergistic interaction between the two drugs.
References
- Aksit D, Yalinkilinc SH, Sekkin S (2015) Comparative pharmacokinetics and bioavailability of albendazole sulfoxide in sheep and goat, and dose-dependent plasma disposition in goats. BMC Vet Res 11: 124.
- Aliu YO (2007) Veterinary Pharmacology, 1st, Tamazzan Publishing Company Limited, Zaria.
- Capece BPS, Castella G, Perez F, Arboix M, Cristofol C (2000) Pharmacokinetic behavior of albendazole sulfoxide enantiomers in male and female sheep. Vet Res Commun 24(5): 339-348.
- Prescott JF (2000) Antimicrobial Therapy in Veterinary Medicine, 3rd Ames, Iowa, Blackwell, Iowa.
- Barragry TB (1994) Veterinary Drug Therapy, Lea and Febiger, Philadelphia, USA.
- Nouws JFM, Vree TB, Aerts R, Grodel J (1986) Pharmacokinetics and residues of sulphadimidine, its N4-acetyl and hydroxyl metabolites in food producing animals. Archiv für Lebensmittelhygiene 37: 57-84.
- Akogwu EI, Saganuwan, SA, Onyeyili, PA (2017) Effects of Piroxicam on Pharmacokinetics of Sulphadimidine in West African Dwarf Male and Female Goats (Capra hircus). Pharm Anal Acta 8: 555.
- Elsheikh HA, Ali BH, Homeida AM (1991) Pharmacokinetics of antipyrine and sulphadimidine (sulfamethazine) in camels, sheep and goats. J Vet Pharmacol Therap 14(3): 269-275.
- Onyeyili PA, Ogundele OO, Sanni S (2000) Effect of starvation on the elimination kinetics of sulphadimidine in Broiler chickens. Nig J Exper Appl Biol 1: 25-28.
- Agbo JO, Saganuwan SA, Onyeyili PA (2016) Comparative Pharmacokinetics of Intramuscular Sulphadimidine in Non-starved and Starved Grower Turkeys (Meleagris gallopavo). J Pharmacol Toxicol 11: 11-19.
- Onyeyili PA, Egwu GO, Apampa OA, Ameh J (1997) Elimination of sulphadimidine from edible tissues and blood of guinea fowl, domestic chickens and ducks. Bull Afri Anim Hlth Prod 45: 225-229.
- Saganuwan SA, Elsa AT, Mahammad BY (2003) Disposition kinetics of sulphadimidine in Nigerian mongrel dogs. J Sci Indust Stud 1(2): 35-38.
- (2018) ICLAS and CIOMS. Internation guilding principles for biomedical research involving animals.
- Nagaraja P, Naik SD, Shrestha AK (2007) A sensitive spectrophotometric method for the determination of sulfonamides in pharmaceutical preparations. Acta Pharm 57(3): 333-342.
- Saganuwan SA (2012) Principles of Pharmacological Calculations, Ahmadu Bello University Press, Zaria.
- Baggot JD (2001) The Physiological Basis of Veterinary Clinical Pharmacology, Blackwell Science, Oxford, UK.
- Nielsen P, Rasmussen F (1977) Half-life, volume of distribution and protein binding for some sulphonamides in cows. Vet Res Sci 22(2): 205-208.
- Nawaz M (1980) Pharmacokinetics and dosage of sulphadimidine in dogs. J Vet Med 26: 75-80.
- Atef MM, Elsayed MCA, Ramadan A (1981) Pharmacokinetics of some sulphonamides in buffaloes. Zentralbl Veterinamed 28(2): 122-130.
- Saganuwan SA (2020) Conversion of benzimidazole, imidazothiazole and imidazole into more potent CNS acting drugs. Cent Nerv Syst Agents Med Chem 20(1): 3-12.
- Elsheikh HA, Osman IAM, Abdullah AS (1997) The effect of water deprivation on the pharmacokinetics of antipyrine and sulphadimidine following intravenous administration in Nubian goats. Vet Res Commun 21(8): 587-597.
- Eduardo EB, Diego CD, Eduardo P (2008) Effects of age on the pharmacokinetics of single dose sulfamethazine after intravenous administration in cattle. Vet Res Comm 32(7): 509-519.
- Youssef SA, El-Gendi AYI, El-Sayed MGA (1981) Some pharmacokinetic and biochemical aspects of sulphadiazine and sulphadimidine in ewes. J Vet Pharmacol Ther 4(3): 173-182.
- Nilsson EP, Yosikawa T, Oka C (1960) Quantification of antibiotics using HPLC, Tetracycline. Antimicrobial Agents and Chemotherapy 9: 745-760.
- Srivastava AK, Rampal S (1990) Disposition kinetics and dosage regimen of sulphamethazine in sheep (Ovis aries). British Veterinary Journal 146: 239-242.
- Bevil RF (1982) Sulphonamides. In: Jone’s Veterinary Pharmacology and Therapeutics. N.H. Booth and L.E. MacDonald, eds., Kalyani Publications, New Delhi, India.
- Bywater RJ (1982) Veterinary Applied Pharmacology and Therapeutics 4th edn, Bailliere Tindal, London.






























