LC-MS/MS Application for Bioequivalence of Cefixime in Human Plasma
Ahmed Abu-awwad1,2*, Khaled W Omari3, Basel Arafat4*, Eyad Mallah5, Mona Bustami5 and Tawfiq Arafat2
1Faculty of Pharmacy, Jerash University, Jordan
2Jordan Center for Pharmaceutical Research, Jordan
3College of Engineering and Technology, American University of the Middle East, Kuwait
4Faculty of Health, Education, Medicine and Social Care, Anglia Ruskin University, Chelmsford campus, UK
5Faculty of Pharmacy and Medical Sciences, University of Petra, Amman –Jordan
Submission: November 19, 2022; Published: November 28, 2022
*Corresponding author:Ahmed Abu-awwad, Faculty of Pharmacy, Jerash University, Jordan
How to cite this article:Ahmed A-a, Khaled W O, Basel A, Eyad M, Mona B, et al. LC-MS/MS Application for Bioequivalence of Cefixime in Human Plasma. J of Pharmacol & Clin Res. 2022; 8(5): 555750. DOI: 10.19080/JPCR.2022.08.555750
Abstract
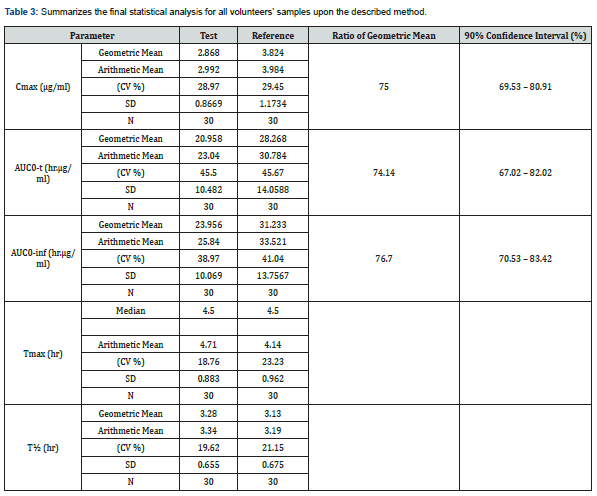
A new bioanalytical method was developed and validated for determination of cefixime in human plasma using LC-MS/MS technique, then successfully applied to bioequivalence study for evaluation of test product against the reference product of Cefixoral® 400 mg film-coated tablet, following comparative randomized, single dose, two-period cross over design, in thirty healthy male subjects, under fasting conditions. Cefixime with labeled internal standard (Cefixime [13C,15N2]) was extracted from plasma by protein direct precipitation in single extracting step, and separated on ACE C18 column (4.6 x 50 mm, 5 μm) by mobile phase of 50% methanol in 10.0 mM ammonium formate and delivered isocratically at flow rate 0.7 ml/min. The established method was fully validated over dynamic range of 0.07-7.0 μg/ml according to the European guideline for bioanalytical methods validation, where all the test’s results were within the acceptance criteria. The investigated pharmacokinetic parameters of Cmax and AUC0-24 were 3.824 ug/ml and 28.268 hr.μg/ml, respectively, while the corresponding values for test product were 2.868 ug/ml and 20.958 hr.μg/ml, respectively. In conclusion, the test product was not bio comparable to the reference product accordingly.
Keywords: Cefixime; Bioequivalence; LC-MS/MS; Human plasma; Plasma precipitation
Abbreviations: PK: Pharmacokinetic; IS: Internal Standard; QC: Quality Control; JCPR: Jordan Center for Pharmaceutical Research
Introduction
Cefixoral® (Cefixime C16H15N5O7S2.3H2O); (Figure 1) is a semisynthetic, cephalosporin antibacterial for oral administration. Chemically, it is (6R,7R)-7-[2-(2-Amino-4-thiazolyl) glyoxylamido]-8-oxo-3-vinyl-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylic acid, 72-(Z)- [O- (carboxy methyl) oxime] trihydrate. Cefixime as antibiotic is indicated in the treatment of the infections caused by susceptible strains of the microorganisms that cause acute uncomplicated cystitis and urethritis in urinary tract, otitis media, pharyngitis and tonsillitis and acute exacerbations of chronic bronchitis uncomplicated cervical or urethral gonorrhea [1]. The pharmacokinetic (pk) parameters for cefixime post oral administration of film-coated tablet 400 mg under fasting conditions have evaluated in youth and elderly male subject, where Cmax was around 3.88 μg/ml, Tmax 3.7 hr and AUC0-24 28.2 hr.μg/ml in youth [2], and the corresponding pk parameters were higher in elderly [3].
Cefixime has long been evaluated in human plasma by HPLC [4,7], while a very limited studies investigated cefixime by LC-MS/MS [8,9], where such technique provides more sensitive and selective results, but in less availability among research facilities. Herein, we designed in current study to use LC-MS/MS technique in the evaluation of bioequivalence of cefixime using newly developed and fully validated method in order to provide a supporting pk date that agree with the previous studies [2] which applied the corresponding cefixime dose on healthy adult and youth subject, where our pk finding data were a little lower (around 20%) than what are reported pk data in elderly subjects [3]. Furthermore, in current conclusion we provide a recommendation for the non-bio comparable test product, in order to enhance the bioavailability with comparative to the reference product.
Experimental
Chemicals and reagents
Cefixime reference standard used in study (purity = 88%) and internal standard (IS) Cefixime [13C,15N2] (purity = 90%) were obtained from TRC Inc. (Toronto, Canada). The blood blank samples were harvested from donors and collected in the clinical site, then plasma separated immediately for using in validation.
LC/MS-quality deionized water, methanol, acetonitrile, ammonium formate and ethyl acetate were purchased from Fisher, Germany, and the other chemicals were all of analytical grade.
Instrumentation
The Mass spectrometer was API 4000, Applied Biosystems, MDS SCIEX, coupled to LC from Agilent 1200 series. Computer System of Windows 7 SP1, and Analyst 1.6.3 software for data management system.
LC Conditions
The chromatographic conditions were consisted of mobile phase of 1.0 mM Ammonium formate: methanol (1:1 %, v/v), pumped isocratically through column of ACE C18 (50 x 4.6) mm, 5 μm, at constant flow rate of 0.7 ml/min under fixed temperature of 40 °C for column oven and samples tray temperature fixed at 15 °C where the injection volume was 10 μl and total run time of 1 min.
Mass spectrometric conditions
The optimized mass spectrometric conditions for MRM were DP 50, EP 5.3, CE23.8 and CXP 16. The ion source conditions were curtain gas = 12, CAD = gas 10, gas1 = 55, gas1 = 55, gas2 = 40, evaporation temperature = 600 °C and the ion source voltage = 5500 V under positive scan mode.
Standard solution
A stock (Master) solution of Cefixime (1.0 mg/ml) was prepared by weighing accurately equivalent to 10 mg of Cefixime in 10 mL V.F, added about 7 ml of MeOH, vortexed until dissolved, completed to volume with MeOH. A stock (Master) solution of Cefixime [13C,15N2] (1.0 mg/ml) was prepared by dissolving with equivalent volume of MeOH and got a concentration (1.0 mg cefixime [13C,15N2]/ml). A 1000 μl of cefixime master solution (1.0 mg/ml) diluted into 10 ml of diluent. The resultant concentration was (100.0 μg cefixime/ml working solution). A 500 μl of cefixime [13C,15N2] master solution (1.0 mg/ml) diluted into 50 ml of diluent. The resultant concentration was (10.0 μg cefixime [13C,15N2] /ml working solution).
Calibrators and QCs’ Aliquots:
Calibrators and quality control samples (QC) used during study samples analysis were prepared using human heparinized blood and spiked with prepared working solutions. The aliquots were tested for interference and stored in freezer under controlled temperature of -20 °C.
Standard calibration curves and quality control samples: Standard calibrators and quality control samples for cefixime in human plasma (analyte-free pooled plasma) were prepared by spiking 50 μl of working solution into 450 μl of plasma to prepare the calibrators of 0.07, 0.15, 0.30, 0.60, 1.20, 2.40, 4.80 and 7.00 μg/ml. Four QC samples of each four concentrations (0.210, 0.840, 2.800 and 5.600) μg/ml gathered in one set and processed among the batch samples. The proposed dynamic range of the calibration curve was following the European [10] and US FDA [11] guidelines for bioequivalence studies and upon maximum concentration of around 4 ug/ml cefixime in human blood [2].
Sample preparation
A 200 μL volume of plasma was transferred to a 1.5 ml polypropylene tube, where 200 μL of 5% w/v trichloroacetic acid contains I.S was added. The mixture was vortexed for 30 seconds using a Vibrax Type VX-Z, VXR Basic Vortexer (IKA-Werke GmBH & Co. Staufen, Germany) and centrifuged using Multitude Sigma1-15 (Sigma, Germany) for 5 min at 14000 rpm. The supernatant was transferred to an auto sampler micro vial and 2 μL was injected into the analytical column.
Bioanalytical method validations
The developed method for investigation of cefixime in human plasma was fully validated in concordance with the European [12] and US FDA [13] guidelines for bioanalytical methods validation. The method was validated in terms of specificity, LLOQ, carryover, sensitivity, response linearity, accuracy, precision, dilution integrity, matrix effect, recovery and stability.
Clinical design
This study was conducted in compliance with declaration of Helsinki according to GCP and GLP guidelines [14]. The protocol was approved by the local institutional review board. A written informed consent and consent form were obtained from all participant volunteers before proceeding study. This study was designed an open label, randomized, single oral dose, two periods, two sequences, two treatments, laboratory-blinded, crossover study, under fasting conditions. The dosage of test and reference products were not related to analytical shortages, but to the necessity to authorize the 400 mg dosage form according to international regulations. Reference: Cefixoral® 400 mg film coated tablet. Each film coated tablet contains 400 mg Cefixime, manufactured by A Menarini Industrie Farmaceutiche Riunite s.r.l., Italy. 30 subjects were randomized, enrolled, completed and analyzed. In each study period, a (2 x 8 ml) blood samples were collected pre-drug administration and a series of 20 x 7 ml blood samples were collected at the following times: 0.5, 1, 1.5, 2, 2.5, 2.75, 3, 3.25, 3.5, 3.75, 4, 4.5, 5, 5.5, 6, 7, 8, 10, 12 and 24 hours post drug administration.
Result and Discussion
LC–MS/MS analysis
The optimized tandem MS’s parameters exhibited a high quantitative detection efficiency, where the molecular ion for cefixime was detected with its daughter fragment at m/z 453.9/126.1 and 456.9 /129.1 for IS.
Method validation results
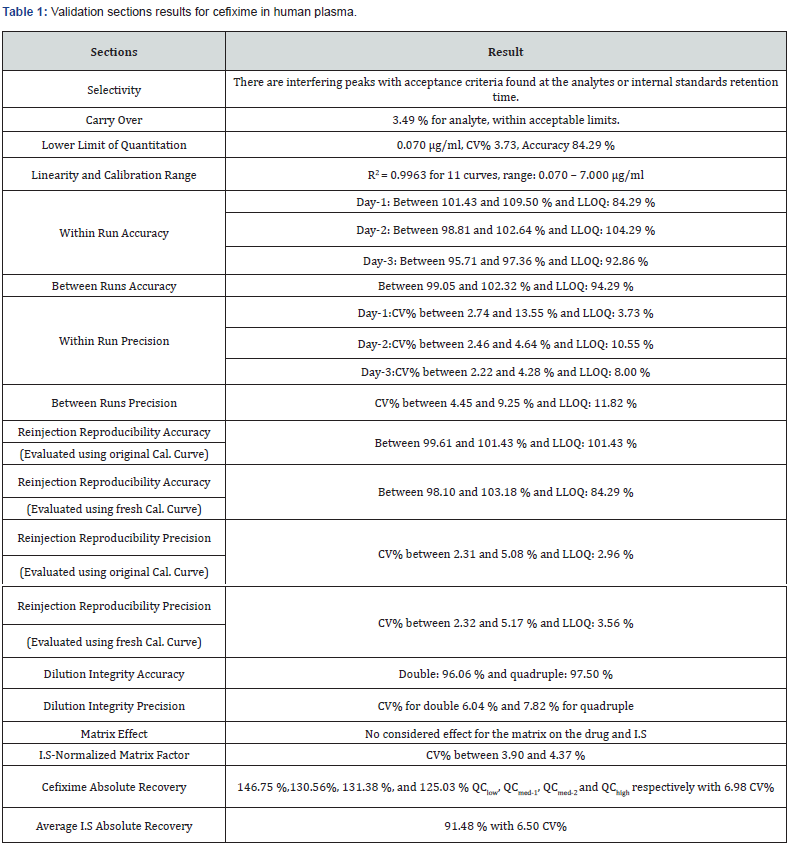
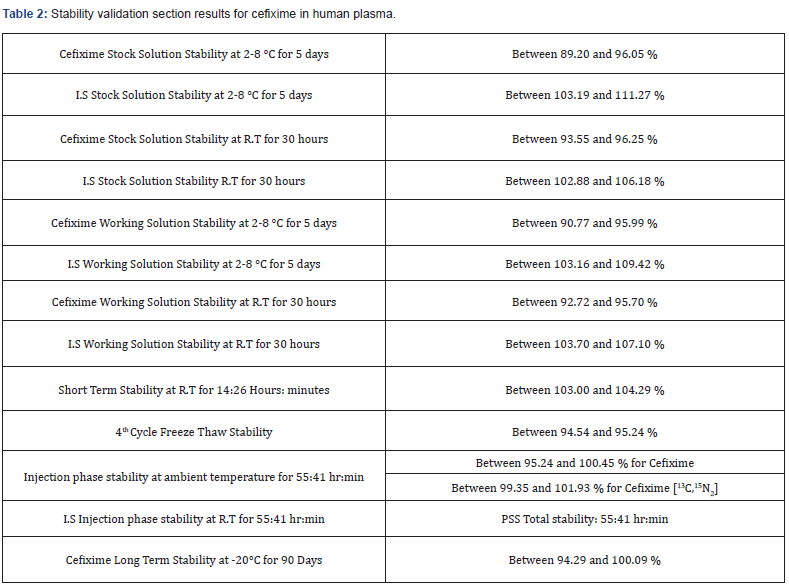
The used extraction method from human plasma was specific and accurate enough to quantitate cefixime over IS properly, and no endogenous peaks observed through validation and routine analysis (Table 1). Summarizes the results obtained from the achieved validation sections, and (Table 2) shows the stability test results.
Clinical application
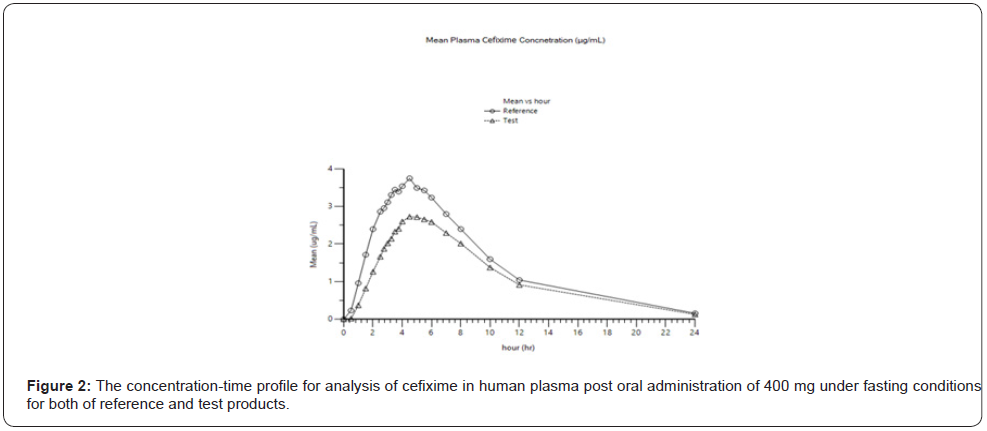
During clinical application, there were no clinically relevant abnormalities at physical examination, and all findings were normal for all volunteers, and there were no safety concerns during the course of the study. Table 3 summarizes the final statistical analysis for all volunteers’ samples upon the described method. The illustration of concentration-time profile for analysis of cefixime in human plasma post oral administration of 400 mg under fasting conditions is presented in (Figure 2). for participant volunteers treated either by test or reference product.
Conclusion
The described method for determination of Cefixime in human plasma by tandem MS was successfully validated and used to estimate a clinical bioequivalence of 400 mg Cefixime on healthy adult and youth male subjects under fasting conditions. The test product of 400 mg capsule and Cefixoral® 400 mg film coated tablet, each dose contains 400 mg Cefixime are not bioequivalent with regard to Cmax and AUC0-t. Herein, the recommendation generic manufacturer is to decrease the particle size of the used raw powder material.
References
- (2018) Auro Pharma Inc, Product monograph Pr Auro-Cefixime p: 1-55.
- Faulkner RD, Bohaychuk W, Haynes JD, Desjardins RE, Yacobi A, et al. (1998) The pharmacokinetics of cefixime in the fasted and fed state. Eur J Clin Pharmacol 34(5): 525-528.
- Faulkner RD, Yacobi A, Barone JS, Kaplan SA, Silber BM (1987) Pharmacokinetic profile of cefixime in man. Pediatr Infect Dis J 6(10): 963-970.
- Liu GL, Sha RG, Gao S, Shen YX, Wang SX (1993) [Determination of cefixime in human plasma and urine using high performance liquid chromatography column switching technique]. Yao Xue Xue Bao 28(3): 216-221.
- Pisarev VV, Zaĭtseva KV, Smirnova LB, Belolipetskaia VG, Kibal’chich DA, et al. (2009) [Determination of cefixime plasma plasma levels by HPLC]., Antibiot. i khimioterapiia = Antibiot. chemoterapy [sic] 54: 37-40.
- Yamazaki H, Inoue K, Hashimoto M, Shimada T (1999) Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Arch Toxicol 73(2): 65-70.
- Khan A, Iqbal Z, Khan MI, Javed K, Khan A, et al. (2011) Simultaneous determination of cefdinir and cefixime in human plasma by RP-HPLC/UV detection method: Method development, optimization, validation, and its application to a pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci 879(24): 2423-2429.
- Attimarad MV, Alnajjar AO (2013) A conventional HPLC-MS method for the simultaneous determination of ofloxacin and cefixime in plasma: Development and validation. J Basic Clin Pharm 4(2): 36-41.
- Dubala A, Nagarajan JSK, Vimal CS, George R (2015) Simultaneous liquid chromatography-mass spectrometry quantification of cefixime and clavulanic acid in human plasma. J Chromatogr Sci 53(5): 694-701.
- European Medicines Agency, European Medicines Agency, Guideline on bioanalytical method validation.
- FDA C, U.S.D. of H. and H. Services, F. and D.A. FDA (2001) Food and Drug Administration, F. and D. Administration, Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services. Food Drug Adm Cent Drug Eval Res (CDER), Cent. Vet. Med.
- (2019) C. for H.M. Products, ICH guideline M10 on bioanalytical method validation, in: EMA/CHMP/ICH.
- (2018) Food and Drug Administration, Bioanalytical Method Validation Guidance. Food Drug Adm 1043: 25.
- (2013) Declaration of Helsinki 2013, Declaration of Helsinki, Ethical Principles for Scientific Requirements and Research Protocols. World Med Assoc p: 29-32.






























