Effect on the Expression of Serum Cytokines after Administration of Biofield Treated Proprietary Test Formulation in TNBS-induced Ulcerative Colitis Rats
Mahendra Kumar Trivedi1 and Snehasis Jana2*
1Trivedi Global, Inc., USA
2Trivedi Science Research Laboratory Pvt. Ltd., India
Submission: May 27,2021; Published: June 15, 2021
*Corresponding author:Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Thane-West, Maharashtra, India
How to cite this article:Mahendra Kumar T, Snehasis J. Effect on the Expression of Serum Cytokines after Administration of Biofield Treated Proprietary Test Formulation in TNBS-induced Ulcerative Colitis Rats. J of Pharmacol & Clin Res. 2021; 8(4): 555745. DOI: 10.19080/JPCR.2021.08.555745
Abstract
The study was aimed to evaluate the gut health protective effect of Biofield Treated/Blessed proprietary test formulation on serum cytokines. Each constituents of the novel test formulation was divided into two parts, one part was denoted as control and the other part was defined as Biofield Energy Treated/Blessed test formulation, which received Biofield Energy Healing Treatment/Blessing (prayer) remotely for about 3 minutes by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. The serum was subjected to test the following biomarkers viz. C-reactive protein (CRP), myeloperoxidase (MPO), and pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-10, IL-23, interferon gamma (IFN-γ), and transforming growth factor beta (TGF-β) using ELISA method. CRP level was decreased by 17.12%, 40.13%, 34.79%, 42.28%, and 12.88% in the G5 (Biofield Treated test formulation), G6 (Biofield Energy Treatment per seat day -15), G7 (Biofield Treated test formulation from day-15), G8 (Biofield Treatment/Blessing per se to the animals with Biofield Treated/Blessed test formulation from day -15), and G9 (Biofield Treatment/Blessing per se from day -15 to animals plus untreated test formulation) groups, respectively as compared with the disease control (G2) group. TGF-β level was decreased by 9.51% and 9.99% in the G7 and G8 groups, respectively than untreated test formulation (G4) group. In addition, the level of IL-23 was decreased by 22.86%, 30.61%, 25.69%, 42.15%, and 25.81% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2. Further, the level of IL-6 was significantly decreased by 54.4% (p≤0.05), 30.5%, 20%, and 48% (p≤0.05) in the G5, G6, G7, and G8 groups, respectively with respect to the G4 group. There was an alteration of TNF-α compared to G2. Besides, IL-10 level was increased by 138.63%, 169.26%, 106.07%, 102.79%, and 81.55% in G5, G6, G7, G8, and G9 groups, respectively as compared with the G2 group. However, the serum MPO level was significantly decreased by 21.33% (p≤0.05), 15.42%, 22.61% (p≤0.05), 13.21%, and 26.25% (p≤0.05) in G5, G6, G7, G8, and G9 respectively, than G2 group. The Trivedi Effect®-Consciousness Energy Healing/Blessing (prayer) would be the best alternative approach for treating gut inflammatory disorders. Thus, the results showed the significant retard the inflammation-related to gastrointestinal diseases (Crohn’s disease, ulcerative colitis, along with microbial, parasitic, and viral inflammatory disorders), and its complications in the preventive treatment groups (viz. G6, G7, G8, and G9).
Keywords: Gut health; Cytokines; Biofield Energy Healing; Inflammatory Disorders; The Trivedi Effect®
Introduction
One of the best defence mechanisms against environmental aggression is the inflammation, which is considered as the most common type of human body response. However, the inflammation frequency and the degree of inflammation varied and depend upon the size of the affected tissues. Inflammatory Bowel Diseases (IBDs) included loss of immune tolerance towards intestinal flora, which is mediated by various cytokines [1]. Gastrointestinal (GI) tract is considered as one of the most susceptible body tissues because it is continuously exposed to various forms of antigens, mutagenic, and toxic factors. Cytokines play a major role in IBDs pathogenesis, which are the intestinal immune response. These cytokines have been found to have multiple roles, which control various aspects of the inflammatory responses. However, it has been found in various IBDs that imbalance between the pro-inflammatory and anti-inflammatory cytokines disrupt the resolution of inflammation that ultimately results in perpetuation of the diseases and tissue destruction.
Thus, the identification of new cytokines, specific cytokines, or anti-cytokine antibodies, which are responsible for IBDs can be the new target for advanced new drug therapies such as in Crohn’s disease and ulcerative colitis. Cytokines also responsible for the cellular communication among the cells, cause proliferation of the antigen specific effectors cells, and regulates local and systemic inflammation using different pathways [2,3]. Adaptive and innate immune responses are the major responses in gut inflammation. Cytokines also regulates the alteration of T-cell activity, and in gut inflammation overproduction or dysregulation of T-cells occurs, which leads to various forms of IBDs. Thus, cytokines and the T-cells have significant complex action on the inflammatory gut diseases [4]. These cellular interactions are regulated using different both pro or anti-inflammatory cytokines (TNF-α, INF-γ, IL-1, IL-6, IL-4, IL-5, IL10, and TGF-β) and various cytokines such as IL-13, IL-12, IL-18, and IL-23) [5-10]. In order to mediate the gut inflammation, novel test formulation was designed to alter the serum cytokines for its significant role in various inflammatory diseases conditions. The novel test formulation comprised of zinc chloride, ferrous sulphate, copper chloride (II-cupric), pyridoxine HCl (vitamin B6), cyanocobalamin (vitamin B12), magnesium (II) gluconate, and cholecalciferol (vitamin D3) and it was tested against the inflammatory cytokines in gut health.
“Biofield Therapy” is regarded as the CAM approach and it has been reported with various therapeutic outcomes. Biofield Energy Therapy against various chronic cases like immune disorders and the patients with cervical cancer patients has been reported with significant benefits [11,12]. National Center for Complementary/Alternative Medicine (NCCAM) has defined and recommended the CAM therapies, which has been exist in various forms of therapies like Reiki, Qi Gong, Johrei, Tai Chi, Rolfing structural integration, therapeutic touch, healing touch, guided imagery, yoga, Ayurvedic medicine, polarity therapy, chiropractic/ osteopathic manipulation, pranic healing, deep breathing, pilates, meditation, massage, progressive relaxation, traditional Chinese herbs and medicines, homeopathy, hypnotherapy, acupressure, acupuncture, movement therapy, special diets, mindfulness, in biological systems both in vitro and in vivo. “Biofield Energy” is a subtle energy, which has various clinical benefits [13,14]. The Trivedi Effect®- Consciousness Energy Healing/Blessing (prayer) has been studied and were reported with numerous significant outcomes in the reputed journals in the field of materials science [15,16], agriculture science [17], antiaging [18], Gut health [19], nutraceuticals [20], pharmaceuticals [21], cardiac health [22], bioavailability [23] overall human health and wellness. Thus, the present work was proposed to study the effect of the test formulation on serum cytokines that could significantly helped to improve the prevalence of gut inflammatory diseases using TNBS (Tri-nitro benzene sulfonic acid)-induced ulcerative colitis in male Sprague Dawley rats.
Materials and Methods
Chemicals
Cholecalciferol (vitamin D3), copper chloride, sulphasalazine, iron (II) sulfate, and sodium carboxymethyl cellulose (Na-CMC) were obtained from Sigma-Aldrich, USA. Cyanocobalamin (vitamin B12), zinc chloride, magnesium (II) gluconate, and pyridoxine hydrochloride (vitamin B6) were procured from TCI, Japan. TNBS (Tri-nitro Benzene Sulphonic acid) was obtained from Hi Media, India. Other chemicals used in this study were analytical grade and purchased from India.
Experimental animals
The male Sprague Dawley (SD) rat’s weights approximately 220-350 gm was used, animals were purchased from M/s. National Institute of Biologicals, India. All the animals were randomly divided into nine groups (eight animals in each group) according to their body weights. They were kept individually in sterilized polypropylene cages with stainless steel top grill with capacities of holding feed and drinking water bottle. The animals were maintained as per standard protocol throughout the experiment.
Consciousness energy healing treatment
Each ingredient of the test formulation used in this study was divided into two parts. One part of each ingredient was considered as the control and no treatment was given, while the second part of each ingredient received Biofield Energy Treatment/Blessing by Mr. Mahendra Kumar Trivedi (known as the Trivedi Effect®) under laboratory conditions for ~3 minutes. In addition, three different test groups as per experimental protocol, the animals were also received Biofield Energy Treatment/Blessing (prayer) under laboratory conditions for ~3 minutes. The energy transmission was done to the samples or animals remotely. Similarly, the control samples were subjected to “sham” healer under the same laboratory conditions for ~3 minutes. The “sham” healer did not have any knowledge about the Biofield Energy Treatment/ Healing. After that, the Biofield Energy Treated/Blessed samples were kept in the similar sealed condition. The Biofield Energy Treated/Blessed animals were also taken back to the animal experimental room for further proceedings.
Experimental test groups
The serum cytokines along with CRP and level of MPO was estimated using standard experimental methods. The test groups (G) were divided into 9 groups i.e. denoted as G1 to G9. G1 denoted as normal control with vehicle (0.5% CMC), G2 group defined as colitis control, with 50% TNBS in ethanol using intra colonic route, G3 group included reference compound i.e. sulfasalazine (250 mg/kg), G4 group included administration of untreated test formulation, G5 included Biofield Energy Treated test formulation, G6 group denoted as Biofield Energy Treatment per se to the animals (Day -15) along with vehicle (0.5% CMC), G7 group defined as Biofield Energy Treated test formulation from day -15, G8 group included Biofield Energy Treatment per se to the animals along with Biofield Energy Treated test formulation from day -15, and G9 group animals were administered with the Biofield Energy Treatment per se (day -15) to the animals along with the untreated test formulation. 50% TNBS in ethanol was given to the entire test groups except G1.
Experimental procedure
After acclimatization of the animals, random grouping was performed based on their body weights. Groups viz. G1, G2, and G6 were treated with 0.5% w/v CMC-Na in distilled water for 8 weeks (Day 1 to 56). Group 3 was treated orally with sulphasalazine (reference item) at a dose of 250 mg/kg body weight for 8 weeks. The freshly prepared suspension of the untreated and Biofield Energy Treated test formulation were administered orally to the G4 and G5 groups at a dose of 130.52 mg/kg for 8 weeks. Similarly, groups G7 and G8 were treated with the test formulation at a dose 130.52 mg/kg from the day of Biofield Energy Treatment (day -15 to 56), while in group G9, Biofield Energy Treated animals were treated with untreated test formulation for 8 weeks. Before the induction of experimental colitis, a short fasting preceded.
The duration ranged from 12 to 18 hours, while the chronic colitis was induced by intra colonic administration of TNBS-50% ethanol in a total volume of 400 μL, at a dose of 10 mg/rat. TNBS was instilled by a suitable medical-grade polyurethane catheter for enteral feeding approximately 8 cm proximal to the anal verge. Accordingly, TNBS injection was given on day 1, 8, 15, 21, 27, 34, 40, 48, and 54. After the end of the experiment, the serum was collected from each animal and was used for the estimation of cytokines such as TGF-β, IL-23, IL-6, TNF-α, IFN-γ, and IL-10 along with C - reactive protein (CRP), and MPO.
Assessment of serum cytokines, C-reactive protein (CRP), and myeloperoxidase (MPO)
Serum cytokines were estimated using standard ELISA assay followed by manufacturer instructions. Serum was collected from all the animals after completion of the experiment was examined for level of cytokines, CRP and MPO. The detailed test procedure of the identification of serum cytokines were performed using manufactured instructions as per individual ELISA kit. The ELISA kits used for identification was Ray Biotech for rat IL-6, TNFalpha, IFN-γ, and IL-10, while TGF-β was tested using Enzo ELISA Kit, and interleukin 23 (IL-23) and CRP level was tested using CUSABIO, ELISA Assay Kit as per manufacturer instructions.
Statistical analysis
The data were expressed as mean ± standard error of mean (SEM) and subjected to statistical analysis using Sigma Plot (Version 11.0). For between two groups comparison Student’s t-test was performed, while multiple groups analysis one-way ANOVA was performed followed by post hoc analysis by Dunnett’s test. The p≤0.05 was considered as statistically significant (n=8).
Results and Discussion
Effect of the test formulation on serum C - reactive protein (CRP)
The effect of the novel test formulation and Biofield Energy treatment per se on the level of serum C-reactive protein (CRP) is shown in Figure 1. The serum CRP level in TNBS (G2) treated animals was 176.86 ± 46.85 ng/mL, which was found to be 65.94% higher than that of the normal control (G1) group (106.58 ± 17.18 ng/mL). However, sulphasalazine group (G3) showed reduced serum CRP level (124.64 ± 31.96 ng/mL) by 29.53% as compared with the G2 group. The experimental groups such as the untreated test formulation to the untreated animals (G4) showed reduced CRP level (119.64 ± 15.47 ng/mL) by 32.5% as compared with the G2 group. Similarly, the Biofield Energy Treated test formulation to the untreated animals (G5) reduced the serum CRP level (146.58 ± 22.62 ng/ml) by 17.12% as compared to the G2 group. Biofield Energy Treatment per se to the animals (G6) reduced the CRP level (105.89 ± 17.93 ng/mL) by 40.13% lower as compared to the G2 group.
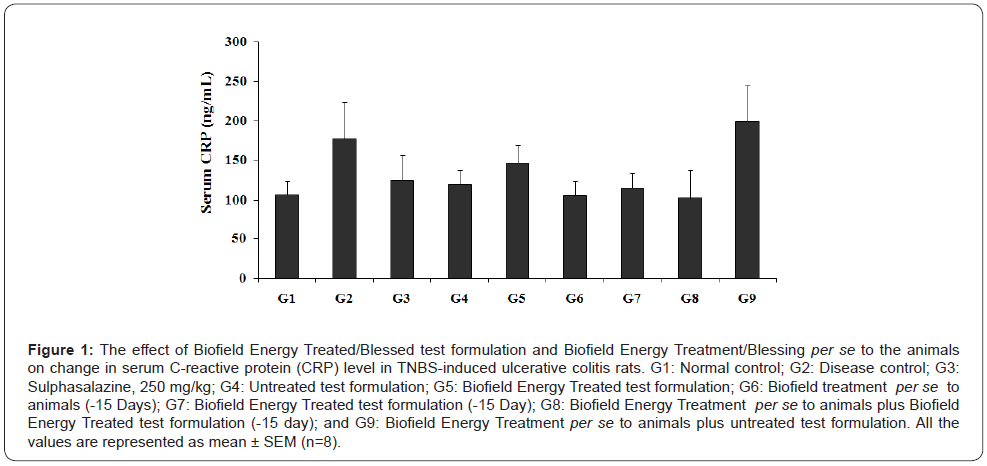
In addition, 15 days pre-treatment of Biofield Energy Treated test formulation (G7) reduced the CRP level (115.33 ± 17.91 ng/ mL) by 34.79% as compared to the G2. Another group, 15 days pre-treatment of Biofield Energy Treated test formulation to the Biofield Energy Treated animals (G8) reduced the CRP level (102.08 ± 35.07 ng/mL) by 42.28% as compared to the G2. Similarly, the untreated test formulation to the Biofield Energy Treated animals (G9) increased the CRP level (199.64 ± 43.90 ng/mL) by 12.88% percentage as compared to the G2. However, data also suggested that the level of serum CRP was decreased by 11.49%, 3.6%, and 14.68% in the G6, G7, and G8 groups, respectively as compared with the untreated test formulation (G4) group. CRP is one of the major inflammatory biomarker (highly sensitive protein) for inflammatory bowel diseases (IBD) [24,25]. Thus, Biofield Energy Treatment per se and the test formulation significantly reduced the serum CRP, which significantly improve the gut inflammatory diseases.
Effect of the test formulation on serum transforming growth factor beta (TGF-β)
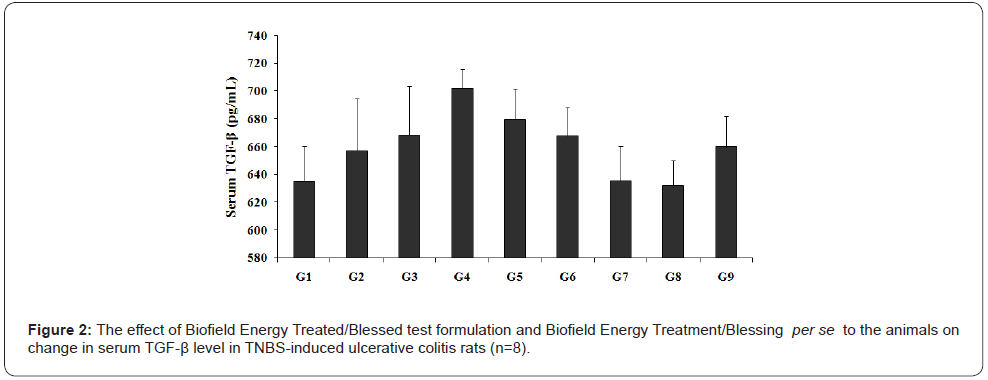
The effect of serum cytokines with respect to transforming growth factor beta (TGF-β) is shown in Figure 2. The level of TGF-β in serum of rats treated with TNBS (G2) was 657.28 ± 37.59 pg/ mL, while the values of TGF-β in the normal control (G1) group was 634.95 ± 25.40 pg/mL. Sulphasalazine treatment (G3) showed the level of TGF-β (668.54 ± 35.04 pg/mL). The TGF-β level among the tested group’s data showed a slight decrease in activity by 3.33% and 3.85% in the G7 and G8 groups, respectively as compared with the G2 group. Further, reduced the level of TGF-β activity was reported by 3.19%, 4.88%, 9.51%, 9.99%, and 6% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the untreated test formulation (G4) group.
Effect of the test formulation on serum interleukin-23 (IL-23)
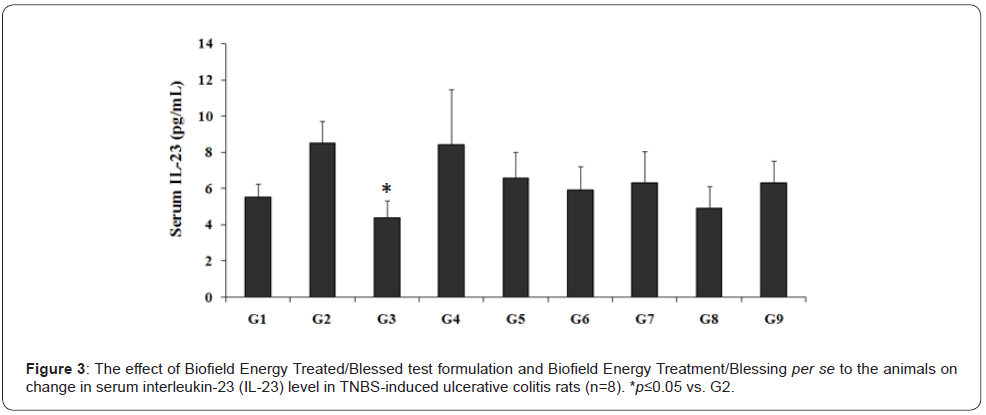
The experimental results of Biofield Energy Treated test formulation and Biofield Energy Treatment per se with respect to the serum level of interleukin-23 (IL-23) are presented in Figure 3. The experimental data suggested that the serum level of IL-23 in animals treated with TNBS (G2) was 8.52 ± 1.21 pg/ mL, which was 53.69% greater than compared with the normal control (G1) group (5.55 ± 0.74 pg/mL). Sulphasalazine treatment (G3) significantly (p≤0.05) reduced the serum IL-23 level (4.41 ± 0.92 pg/mL) by 48.27% as compared with the G2 group. G4 group showed almost similar level of IL-23 as compared with the G2 group. G5 group animal showed a decreased serum IL-23 level (6.57 ± 1.43 pg/mL) by 22.86% as compared with the G2 group. G6 group data showed a decreased IL-23 level (5.91 ± 1.32 pg/ mL) by 30.61% as compared with the G2 group.
Similarly, G7 decreased the level of IL-23 (6.33 ± 1.73 pg/mL) by 25.69% as compared with the G2 group. The level of IL-23 in G8 (4.93 ± 1.18 pg/mL) and G9 (6.32 ± 1.20 pg/mL) was decreased by 42.15% and 25.81%, respectively as compared with the G2 group. Further, the data with respect to the percentage showed a significant decreased the level of IL-23 by 22.04%, 29.87%, 24.9%, 41.53%, and 25.02% in the G5, G6, G7, G8, and G9 groups, respectively with respect to the untreated test formulation (G4) group. IL-23 plays a major role of inflammation in inflammatory bowel diseases (IBDs) and also regulates the healing of diseases [26,27]. Thus, the experimental data suggested significant decreased IL-23 level after Biofield Energy Treatment using novel test formulation, which suggested a novel cause of treatment of gut inflammatory diseases.
Effect of the test formulation on serum IL-6
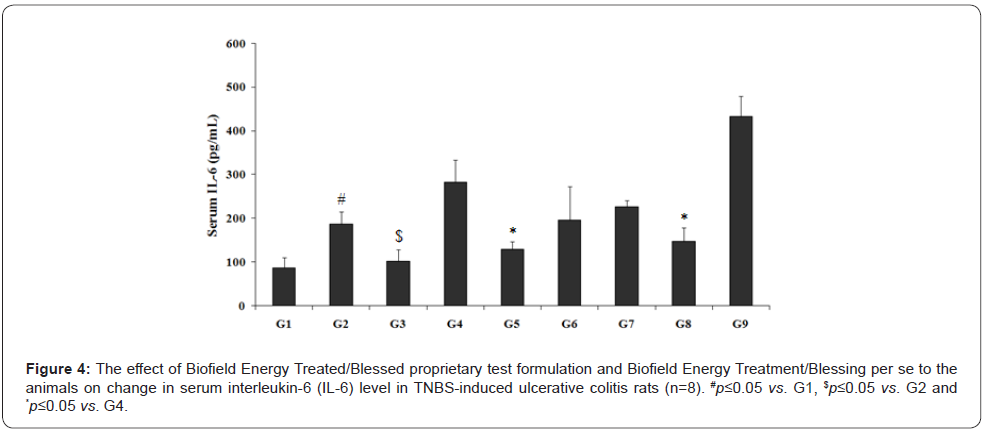
The level of the serum IL-6 data after treatment with the Biofield Energy Treated Test formulation is displayed in the Figure 4. The experimental data suggested that serum IL-6 in TNBS (G2) group was 186.94 ± 28.02 pg/mL, which was significantly (p<0.05) higher than the control (G1) group (86.78 ± 24.66 pg/mL). Sulphasalazine treatment (G3) showed a significantly (p<0.05) decreased the level of IL-6 (101.16 ± 28.30 pg/mL) than G2 group. The experimental test groups such as G5 (128.81 ± 18.56 pg/mL) and G8 (146.94 ± 32.44 pg/mL) showed a decreased IL-6 level by 31.08% and 21.38%, respectively as compared with the disease control (G2) group. Further, the experimental data with respect to the percentage showed a significant decreased serum IL-6 level by 54.4% (p≤0.05), 30.5%, 20%, and 48% (p≤0.05) in the G5, G6, G7, and G8 groups, respectively with respect to the untreated test formulation (G4) group. IL-6 in gut health has been linked with cellular proliferation and repair of intestinal epithelial cells after injury [28] along with its significant contribution against number of autoimmune diseases such as rheumatoid arthritis [29,30].
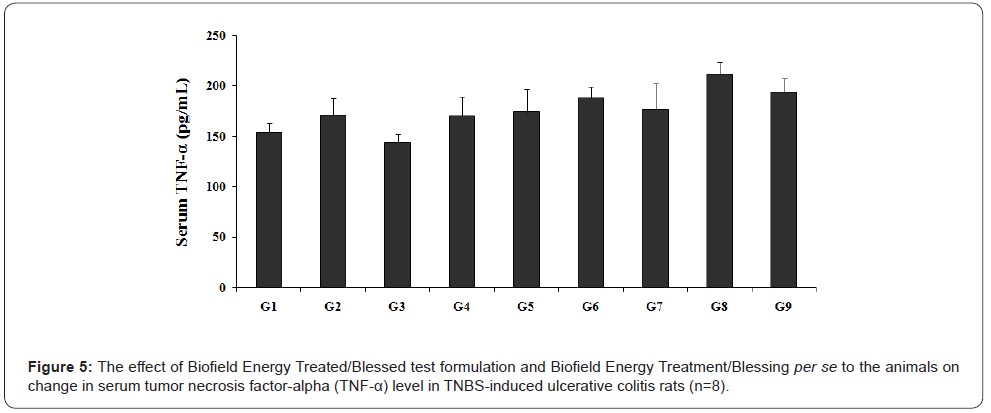
Effect of the test formulation on serum TNF-α
The Biofield Energy Treated test formulation and Biofield Energy Treatment per se to the animals for serum TNF- α is presented in Figure 5. Further, TNF-α level in TNBS (G2) group was 170.67 ± 17.06 pg/mL, which was 10.82% greater than the normal control (G1) group (154.0 ± 8.94 pg/mL). Sulphasalazine treatment (G3) reduced the TNF-α level (144 ± 13.76 pg/mL) by 15.63% as compared with the colitis control group (G2). The TNF-α level and its regulation has been well correlated with number of gut inflammatory diseases like Crohn’s disease [29]. The experimental test groups such as Biofield Energy Treated test formulation showed an alteration of serum TNF-α level as compared with the disease control (G2) and untreated test formulation (G4) groups.
Effect of the test formulation on serum IL-10
The Biofield Energy Treated/Blessed test formulation with respect to the serum level of IL-10 is displayed in Figure 6. The experimental results showed that the level of IL-10 in the serum of rats treated with TNBS (G2) was 1007.43 ± 241.15 pg/mL, which was lower than that of the normal control (G1) group (2286 ± 627.76 pg/mL). Sulphasalazine treatment (G3) increased the level of IL-10 (1244 ± 453.04 pg/mL) by 23.48% as compared to the G2 group. Further, the serum IL-10 level was increased in other experimental test groups such as G4 (2514.86 ± 1437.12 pg/mL) by 149.63%, G5 (2404 ± 1747.89 pg/mL) by 138.63%, G6 (2712.57 ± 1350.15 pg/mL) by 169.26%, G7 (2076 ± 1114.21 pg/ mL) by 106.07%, G8 (2043 ± 653.65 pg/mL) by 102.79%, and G9 (1829 ± 702.34 pg/mL) by 81.55% as compared with the disease control (G2) group.
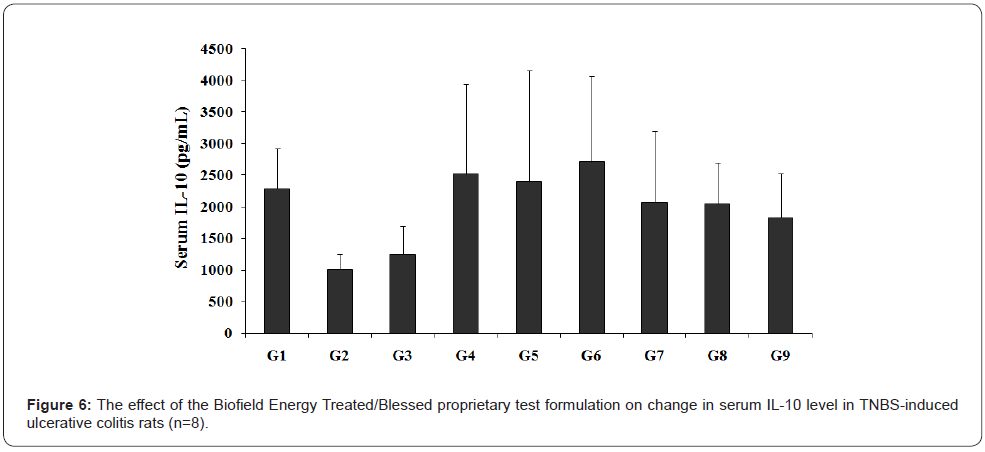
Further, the experimental data suggested that IL-10 level was increased by 7.86% in the G6 group as compared with the untreated test formulation (G4) group. IL-10 indicated a significant antiproliferative effect [31,32]. Besides, it has also the potential role of IL-10 in maintaining the normal non-inflammatory intestinal immunoregulation [33,34], which has significant association with the gut microbiota [33].
Effect of the test formulation on serum myeloperoxidase (MPO)
The experimental data of serum myeloperoxidase (MPO) is presented in Figure 7, and the results showed that TNBS (G2) group data was 16.86 ± 0.81 ng/mL, which was higher than that of the normal control (G1) group (15.84 ± 0.57 ng/mL). Sulphasalazine treatment (G3) significantly decreased (p<0.05) the level of serum MPO (13.16 ± 1.0 ng/mL) by 21.93% as compared to the G2 group. Further, the serum MPO level was significantly decreased in other experimental test groups such as G4 (14.76 ± 0.38 ng/mL) by 12.48%, G5 (13.26 ± 0.42 ng/mL) by 21.33% (p≤0.05), G6 (14.26 ± 0.61 ng/mL) by 15.42%, G7 (13.05 ± 0.77 ng/mL) by 22.61% (p≤0.05), G8 (14.63 ± 1.23 ng/mL) by 13.21%, and G9 (12.43 ± 1.58 ng/mL) by 26.25% (p≤0.05) as compared with the disease control (G2) group. Further, the experimental data suggested that serum MPO level was decreased by 10.13%, 3.39%, 11.6%, and 15.76% in the G5, G6, G7, and G9 groups, respectively as compared with the untreated test formulation (G4) group.
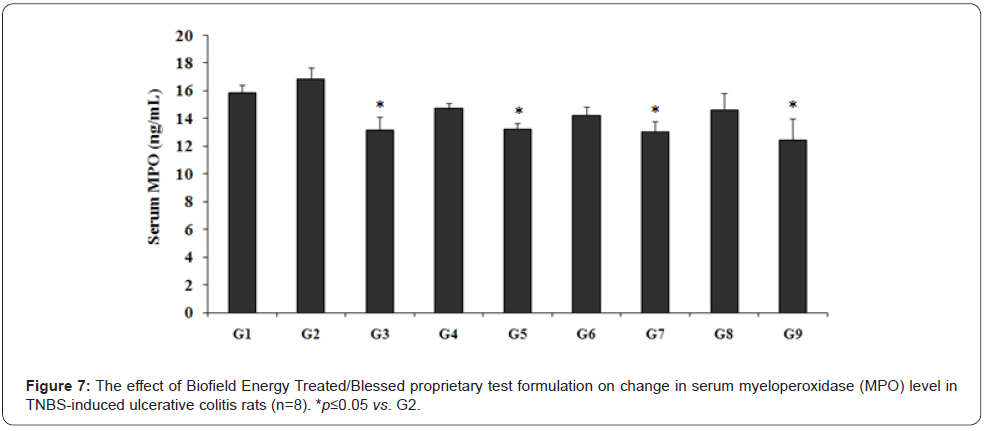
MPO is one of the biomarker in gut inflammation and IBDs and also worked against many gut diseases [35]. Thus, the present experimental data showed a significant change in serum MPO level after Biofield Energy Treatment. Experiment includes four preventive maintenance groups (G6, G7, G8 and G9). The findings showed the significant slowdown of gastrointestinal-related symptoms and also reduced the chances of disease susceptibility. Based on the obtained data, it signifies that Mr. Trivedi’s Biofield Therapy (prayer) was found to be most effective and benefited to protect from the existing disorders or complications that will ultimately improve the overall health and quality of life in human.
Conclusions
Based on the study outcomes it was observed that the serum CRP level was decreased by 40.13%, 34.79%, and 42.28% in the G6, G7, and G8 groups, respectively with respect to the disease control (G2) group. In addition, the serum TGF-β level was decreased by 9.99% in the G8 group as compared with the G2 group. Similarly, the level of IL-23 was decreased by 30.61% and 42.15% in the G6 and G8 groups, respectively than G2. The serum level of IL-6 data showed significantly decreased by 54.4% and 48% in G5 and G8 groups, respectively than untreated test formulation (G4). Serum IL-10 level was increased by 138.63%, 169.26%, 106.07%, 102.79%, and 81.55% in G5, G6, G7, G8 and G9 groups respectively, as compared with the G2 group. MPO, the gut inflammatory biomarker serum level was significantly decreased by 21.33%, 22.61%, and 26.25% in the G5, G7, and G9 groups, respectively than G2.
Experimental results showed a significant gut health activity after treated with the Trivedi Effect®- Biofield Energy Healing.
It could helped to slowdown the gastrointestinal disease progression rate and disease-related complications. Therefore, the Trivedi Effect® might act as a preventive maintenance therapy for the management of overall health and quality of life in human. Biofield Treated test formulation can be used to prevent various inflammatory disorders like inflammatory bowel diseases (IBDs), Crohn’s disease, ulcerative colitis, stress, asthma, etc. Moreover, it can be used against autoimmune disorders viz. Addison disease, Graves’ disease, myasthenia gravis, pernicious anemia, alopecia areata, diabetes, aplastic anemia, fibromyalgia, multiple sclerosis, psoriasis, vitiligo, etc.
Acknowledgement
The authors are gratefully acknowledged to Trivedi science, Trivedi Global, Inc., and Trivedi master wellness and to Dabur Research Foundation (DRF), India for their support.
- Research Article
- Abstract
- Introduction
- Materials and Methods
- Results and Discussion
- Conclusions
- Acknowledgement
- References
References
- Jump RL, Levine AD (2004) Mechanisms of natural tolerance in the intestine: Implications for inflammatory bowel disease. Inflamm Bowel Dis 10(4): 462-478.
- Neuman MG (2007) Immune dysfunction in inflammatory bowel disease. Transl Res 149(4): 173-186.
- Leon F, Smythies LE, Smith PD, Kelsall BL (2006) Involvement of dendritic cells in the pathogenesis of inflammatory bowel disease. Adv Exp Med Biol 579: 117-132.
- Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448(7152): 427-434.
- Papadakis KA, Targan SR (2000) Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 51: 289-298.
- Ince MN, Elliott DE (2007) Immunologic and molecular mechanisms in inflammatory bowel disease. Surg Clin North Am 87(3): 681-696.
- Funk RH, Monsees T, Ozkucur N (2009) Electromagnetic effects-from cell biology to medicine. Prog Histochem Cytochem 43(4): 177-264.
- Bischof M, Del Giudice E (2013) Communication and the emergence of collective behavior in living organisms: A quantum approach. Mol Biol Int 2013: 987549.
- Jain S, Rapgay L, Daubenmier J, Muehsam D, Rapgay L, et al. (2015) Indo-Tibetan philosophical and medical systems: Perspectives on the biofield. Global Adv Health Med 4(Suppl): 16-24.
- Fröhlich H (1968) Long-range coherence and energy storage in biological systems. Int J Quant Chem 2: 641-649.
- Peck SD (1998) The efficacy of therapeutic touch for improving functional ability in elders with degenerative arthritis. Nurs Sci Q 11(3): 123-132.
- Turner JG, Clark AJ, Gauthier DK, Williams M (1998) The effect of therapeutic touch on pain and anxiety in burn patients. J Adv Nurs 28(1): 10-20.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8(6): 703-717.
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Effect of consciousness energy healing treatment on the metal profile and properties of tellurium. Eng Technol Open Acc 3(5): 555623.
- Mahendra KT, Alice B, Dahryn T, Snehasis J (2021) Consciousness energy healing treatment impacted the isotopic abundance ratio of 6-Mercaptopurine (6-MP). Nov Appro Drug Des Dev 5(5): 555673.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica). Journal of Food and Nutrition Sciences 3(6): 245-250.
- Trivedi MK, Jana S (2021) Anti-aging activity of biofield energy treated novel proprietary test formulation by assessment of vital biomarkers in cerebrospinal fluid (CSF) in Sprague Dawley rats. On J Neur & Br Disord 5(2): 2021. OJNBD.MS.ID.000210.
- Trivedi MK, Jana S (2021) Evaluation of biofield energy healing treatment based proprietary test formulation on gut health potential in colon cancer cell line (HT-29). J Pharmacol Clin Res 8(4): 555743.
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Isotopic abundance ratio analysis of consciousness energy healing treated folic acid. Food Nutr Current Res 4(2): 290-295.
- Trivedi MK, Branton A, Trivedi D, Jana S (2020) The consciousness energy healing treatment and its impact on the isotopic abundance ratio analysis of flutamide. Drug Des Int Prop Int J 3(5) - 2020. DDIPIJ.MS.ID.000175.
- Trivedi MK, Jana S (2019) In vitro assessment of the biofield treated test item on cardiac function using rat cardiomyocytes cell line (H9c2) via multiparametric analysis. Journal of Hypertension and Cardiology 2(4): 1-12.
- Trivedi MK and Jana S (2021) Assessment of oral bioavailability of vitamin D3 and its metabolite, 25-hydroxy (OH) vitamin D3 in male Sprague Dawley rats following a single oral dose of biofield energy treated vitamin D3. Bioequiv & Bioavailab Int J 5(1): 000147.
- Vermeire S, Van Assche G, Rutgeerts P (2006) Laboratory markers in IBD: Useful, magic, or unnecessary toys?. Gut 55(3): 426-431.
- Hod K, Ringel-Kulka T, Martin CF, Maharshak N, Ringel Y (2016) High-sensitive C-reactive protein as a marker for inflammation in irritable bowel syndrome. J Clin Gastroenterol 50(3): 227-232.
- Mizoguchi A (2012) Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis 18(9): 1777-1784.
- Monteleone I, Pallone F, Monteleone G (2009) Interleukin-23 and Th17 cells in the control of gut inflammation. Mediators Inflamm 2009: 297645.
- Kuhn KA, Manieri NA, Liu TC, Stappenbeck TS (2014) IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One 9(12): e114195.
- Borruel N, Carol M, Casellas F, Antolín M, Lara F, Espín E, Naval J, Guarner F, and Malagelada JR (2002) Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut 51(5): 659-664.
- Dayer JM, Choy E (2010) Therapeutic targets in rheumatoid arthritis: The interleukin-6 receptor. Rheumatology (Oxford) 49(1): 15-24.
- Bao S, Beagley KW, France MP, Shen J, Husband AJ (2000) Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium Immunology 99(3): 464-472.
- Fukushima K, West GA, Klein JS, Levine AD, Fiocchi C (1993) Opposite modulatory activity of IL-10 and IL-4 on lamina propria mononuclear cells (LpMC) is stimulus-dependent [abstract]. Gastroenterology 104: A702.
- LeBlanc AM, del Carmen S, Zurita-Turk M (2011) Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol 2011: 892971.
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75(2): 263-274.
- Hansberry DR, Shah K, Agarwal P, Agarwal N (2017) Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus 9(1): e1004.






























