Nephroprotective effect of PPAR Agonists on Thioacetamide-induced Nephrotoxicity in Rats
Remon Roshdy Rofaeil1,2*, Ahlam Mohamed Abdellah3 and Nagwa Mostafa Zenhom3
1Department of Pharmacology, Faculty of Medicine, Minia University, Egypt
2Department of Pharmacology, Faculty of Pharmacy, Deraya University, Egypt
3Department of Biochemistry, Faculty of pharmacy, Minia University, Egypt
Submission: February 27, 2019;Published: April 01, 2019
*Corresponding author: Remon Roshdy Rofaeil Department of Pharmacology, Faculty of Medicine, Minia University, Egypt
How to cite this article: Remon R R, Ahlam Md A, Nagwa M Z. Nephroprotective effect of PPAR Agonists on Thioacetamide-induced Nephrotoxicity in Rats. J of Pharmacol & Clin Res. 2019; 6(5): 555698. DOI: 10.19080/JPCR.2019.06.555698
Introduction
Since their isolation by Issemann and Green in 1990, peroxisome proliferator activated receptor (PPAR) became an area of wide and endless interest [1]. To date, three isoforms of PPARs have been identified, namely PPARα, PPARβ/δ, and PPARγ. Importantly, PPARs have been increasingly recognized as key players in the function of many organs including kidney [2]. At the same time, renal diseases became a new epidemic of the twentieth and twenty-first centuries. At present, it is a global problem, mainly because a variety of risk factors is being involved in its etiology and pathophysiology. Although emerging evidence support PPARs may serve as therapeutic targets for treating the nephrotoxicity, there remains a lack of definitive data on their effect on renal functions [3]. There is a controversy about effect of PPAR agonists on renal functions [4,5]. fibrates are PPARα agonists used in treating dyslipidemia, which interplays with renal diseases. Dyslipidemia is a consequence of kidney disease [6] and a large body of clinical and experimental studies support that altered lipid metabolism may contribute to the pathogenesis and progression of kidney disease [7]. telmisartan, an angiotensin type 1 receptor blocker (ARB), is used in treatment of hypertension. In addition, telmisartan has a partial agonistic effect on PPARγ and showed nephroprotective effect in ischemia/reperfusion injury [8]. Worldwide, hypertension is a common cause of end-stage renal disease, which is the last stage of chronic kidney disease [9,10]. the present study aimed to evaluate the effect of PPARα and PPARγ agonists on a model of nephrotoxicity induced in rats by thioacetamide (TA). Furthermore, comparing a possible nephroprotective effect of PPARα agonists versus PPARγ agonists.
Materials and Methods
Drugs and chemicals
Bezafibrate, losartan and telmisartan powder were generously supplied by Egyptian International Pharmaceuticals and Medical Union Pharmaceuticals (Egypt, Cairo) respectively. TA and pyrogallol were purchased from Sigma-Aldrich (USA, Missouri). All other chemicals of analytical grade and were obtained from commercial sources.
Animals
The present study was conducted on adult male wistar rats weighing 205-280 g. Rats were obtained from the animal house, El-Giza, Egypt. Rats were fed a standard diet of commercial rat chow and tap water and left to acclimatize to the environment for one week prior to inclusion in the experiments. All experimental designs were conducted according to the ethical standards approved by the faculty board committee of faculty of medicine, Minia University, Egypt.
Experimental Design
Induction of nephrotoxicity: Nephrotoxicity was induced by intra-peritoneal TA at dose of 50 mg/kg, dissolved in saline 2 ml/kg twice weekly (Monday and Thursday) for 6 weeks. The dose of TA as well as the duration of the study was selected on the light of our pilot experiment and with previous studies [11,12]. All treatments were administered from the first day of TA-intoxication.
Grouping: The animals were randomly divided into 8 experimental groups of 6 animals each. Duration of the study was 6 weeks. (1) Normal control: rats received oral carboxy methyl cellulose (CMC) (p.o.) daily and saline (i.p.) twice weekly; (2) losartan-treated: rats were administered losartan (10 mg/kg, p.o.) [13] suspended in CMC daily and saline (i.p.) was also given twice weekly. (3) Telmisartan-treated: rats were administered telmisartan (10 mg/kg, p.o.) [14] suspended in CMC daily and saline (i.p.) was also given twice weekly. (4) (2) bezafibrate-treated: rats were administered bezafibrate (50 mg/kg, p.o.) [15] suspended in CMC daily and saline (i.p.) was also given twice weekly. (5) TA-treated: TA (50 mg/kg in saline, i.p.) twice weekly and CMC was given daily; (6) TA+Losartan: TA and losartan (10 mg/kg orally) suspended in CMC; (7) TA+Telmisartan: TA and telmisartan (10 mg/kg orally) suspended CMC. (8) TA+Bezafibrate: TA and bezafibrate (50 mg/ kg, p.o.) suspended in CMC.
Sample Collection and Storage
All animals were sacrificed 48 h after the last TA administration. Blood samples were collected and centrifuged at 3000 g for 10 min to obtain clear sera. Kidneys were excised from each rat and then washed by cold saline and divided into parts which were snap frozen in liquid nitrogen, stored at -80 °C, and subsequently homogenized in cold potassium phosphate buffer (pH 7.4) for various biochemical analyses.
Biochemical Analysis
Evaluation of kidney functions: Creatinine and urea levels were determined using commercial kits from Spectrum Diagnostics (Cairo, Egypt).
Renal oxidative stress parameters: Superoxide dismutase (SOD) activity was measured by method of Marklund and Marklund [16] with a slight modification. This method is based on inhibition of the autoxidation of pyrogallol by SOD. The percentage of inhibition for the samples was calculated by the aid of running a control with no sample under the same conditions. SOD enzyme activity was expressed as U/mg protein, where one unit was defined as the amount of the enzyme that inhibited the rate of pyrogallol autoxidation by 50%. Malondialdehyde (MDA), a measure of lipid peroxidation, was evaluated by a method that depends on the reaction between MDA with thiobarbituric and the color developed was measured spectrophotometrically at 535 nm against a blank. Standard curve by 1,1,3,3-tetramethoxypropane was prepared. From this curve, the MDA concentration was expressed as nmol/g tissue then multiplied in the tissue dilution factor [17].
Determination of renal nitric oxide content: The stable oxidation end products of nitric oxide (NO), nitrite (NO2ˉ) and nitrate (NO3ˉ) were measured after the reduction of nitrate to nitrite by copperized cadmium granules. Quantitation of NO2ˉ was based on the Griess reaction and the absorbance of developed color was measured at 545nm against a blank. Concentration of NOx in samples was determined from a standard curve of NaNO3 (0–100 nmol/ml) [18].
Real-time Reverse Transcription Polymerase Chain Reaction for the Relative Quantification of PPARα and PPARγ
Total RNA was extracted from homogenized kidney specimen using ribozol RNA extraction reagent (Amresco, Solon, USA) following the manufacturer’s instructions. cDNAs were synthesized using SensiFAST TM cDNA synthesis Kit (Bioline). cDNA was synthesized at 42 °C for 15 min then at 85°C for 5 min followed by immediate cooling on ice. Realtime polymerase chain reaction (RT-PCR) was performed using 10μL of SYBER Green QPCR Mix (SensiFAST™ SYBER ® Lo-ROX Kit, Bioline). The SYBER green data were analyzed with a relative quantification to GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) as reference gene The sets of primers used were as follows: PPARα sense, 5′- ACGATGCTGTCCTCCTTGATG -3′, and antisense, 5′- GCGTCTGACTCGGTCTTCTTG-3′ PPARγ sense primer; 5ʹ- ATTCTGGCCCACCAACTTCGG -3 ʹ and antisense 5ʹ- TGGAAGCCTGATGCTTTATCCCCA -3ʹ GAPDH sense primers: 5ʹ- GTCGGTGTGAACGGATTTG -3 ʹ and antisense 5ʹ- CTTGCCGTGGGTAGAGTCAT -3 ʹ. The relative expression level of each gene was calculated using the formula 2(-ΔΔCt). They were scaled relative to controls. Thus, results for all experimental samples were graphed as relative expression compared with the control [19].
Statistical analysis
Results were expressed as means ± standard error of mean (SEM). One-way analysis of variance (ANOVA) followed by the Bonferroni’s post analysis test to analyze the results for statistically significant difference. p values less than 0.05 were considered significant. Graph Pad Prism was used for statistical calculations (version 6 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com). The density of PCR product was measured using Scion Image J software (Scio Cooperation, Fredrick, Maryland).
Results
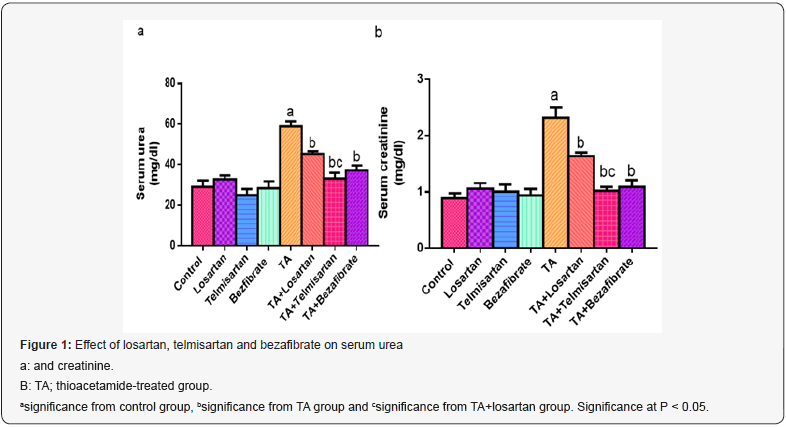
Effect of Telmisartan and Bezafibrate on Serum Urea and Creatinine
Administration of losartan, telmisartan and bezafibrate alone did not produce any significant change in serum urea and creatinine, as compared to control group. At the same time, a significant increase in serum urea and creatinine noticed in TA group, as compared to control group. As compared to TA group, significant reduction in serum urea and creatinine were noticed in groups TA+Losartan, TA+Telmisartan and TA+Bezafibrate but significant reduction occurred in TA+Telmisartan group as compared to TA+Losartan group (Figure 1).
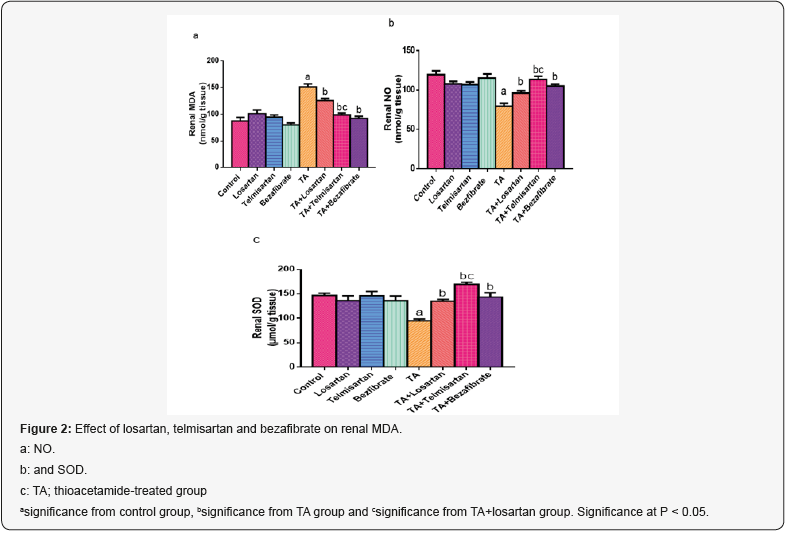
Effect of Telmisartan and Bezafibrate on Renal Malondialdehyde
Similarly, losartan, telmisartan and bezafibrate administration did not produce any significant change in renal MDA, as compared to control group. In TA group, significant increase in renal MDA occurred, as compared to control group. Although significant reduction in renal MDA was noticed in groups TA+Losartan, TA+Telmisartan and TA+Bezafibrate, there was a significant reduction occurred in TA+Telmisartan group as compared to TA+Losartan group (Figure 2).
Effect of Telmisartan and Bezafibrate on Renal Superoxide Dismutase and Nitric Oxide
As compared to control group, administration of losartan, telmisartan and bezafibrate alone did not produce any significant change in renal SOD and NO. Administration of TA caused significant decrease in renal SOD and NO, as compared to control group. In groups TA+Losartan, TA+Telmisartan and TA+Bezafibrate significant increase in renal SOD and NO were noticed, however, significant incresae occurred in TA+Telmisartan group as compared to TA+Losartan group (Figure 2).

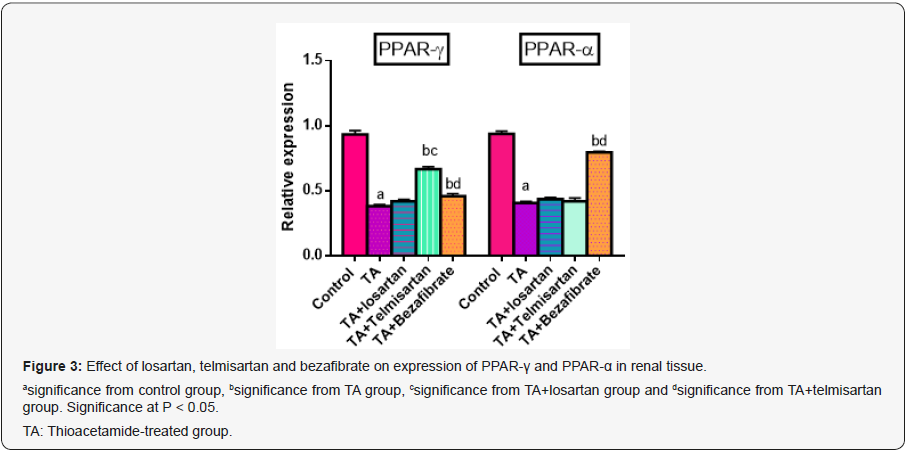
Effect of Telmisartan and Bezafibrate on Renal Expression of PPAR-Γ and PPAR-Α
Administration of TA caused significant decrease in renal PPAR-γ expression, as compared to control group. In groups TA+Telmisartan significant increase in renal PPAR-γ expression were noticed, as compared to TA group, meanwhile, no increase occurred in TA+Losartan and TA+bezafibrate groups. On the other hand, significant decrease in renal PPAR-α expression was noticed in TA group, as compared to control group. At the same time, significant increase in renal PPAR-α expression were noticed in TA+Bezafibrate group but no increase occurred in TA+Telmisartan and TA+losartan groups (Figure 3).
Discussion
Understanding the role of PPAR activation in nephrotoxicity remains a matter of great interest. In the current study, different agonists of PPAR were used to explore the role of PPAR in protection against TA-induced nephrotoxicity. Severe renal damage can be caused by some environmental and industrial toxicants by induction of highly reactive free radicals generation. One of the most extensively studied chemicals and industrial toxicants is TA which is known to induce injury to the terminal portion of the proximal renal tubule. After administration of TA it undergoes an extensive metabolism forming sulfoxide and sulfone which circulate through various organs in body before finally being transformed into acetate and excreted into urine within 24 hours [20].The present investigation revealed that administration of TA for 6 weeks resulted in functional disturbances manifested by a significant increase in serum urea and creatinine levels together with an increase in MDA and reduction in both NO and SOD. As a measure of renal function status, blood urea and creatinine are often regarded as reliable markers of renal damage. Current results agree with previous researches, which reported the nephrotoxic effect of TA and its induction of oxidative stress as seen by increased lipid peroxidation and alteration of antioxidant status [20-22].
Bezafibrate administration was protective against TA-induced nephrotoxicity evidenced by significant decrease in serum urea, creatinine and renal MDA together with increase in both NO and SOD. This effect is accompanied by induction of PPAR-α expression in renal tissue which was reduced with TA treatment. Similar results were obtained referring to the nephroprotective effect of bezafibrate in other models of nephrotoxicity. Those investigators also found the increased expression was associated with reduction in oxidative stress parameters and such reduction was parallel to the nephroprotective effect of fibrates. Also, fibrates ameliorates the apoptotic cell death of renal cells [23,24]. Our study here corroborates the observation that inhibition of PPAR-α expression is linked to nephrotoxic effect of TA, while its induction is linked to nephroprotective effect of bezafibrate.
Telmisartan has dual mechanism of action, an ARB and a PPAR-γ agonist. To clarify the effect of PPAR-γ on this model of nephrotoxicity, we used a selective ARB; losartan. Telmisartan antagonized the development of nephrotoxicity with TA administration. It reduced urea, creatinine and MDA, which was increased with TA. At the same time, it increased NO and SOD in renal tissue that was reduced with TA administration. This protective action was gathered with increased in PPAR-γ expression that was dramatically reduced with TA administration. Our findings agree with above-mentioned studies, which reported the nephroprotective of telmisartan and stated its antioxidant, anti-apoptotic and anti-inflammatory actions in various models of nephrotoxicity [25,26]. At the same time, the protective effect of losartan was significantly lower than the effect of telmisartan indicating the role of PPAR-γ agonistic activity of telmisartan in such nephroprotective effect. In conclusion, our study showed that TA administration caused deterioration in renal function, which was ameliorated by PPAR-α agonist; Bezafibrate and PPAR-γ agonist; telmisartan. The proposed mechanism is antagonizing oxidative stress induced by TA. These findings support the use of fibrates and telmisartan to ameliorate nephrotoxic effect of TA.
Authors’ Contribution
Remon R. Rofaeil carried out study design, performing experiment, biochemical study, analysis, interpretation of data and writing the manuscript. Ahlam K. Abdellah and Nagwa M. Zenhom performed and wrote gene expression part.
References
- Issemann I, Green S (1990) Activation of a member of the steroid hormone receptor super family by peroxisome proliferators. Nature 347:645-650.
- Lapice E, Monticelli A, Cocozza S, Pinelli M, Cocozza S, et al. (2015) The PPARγ2 Pro12Ala variant is protective against progression of nephropathy in people with type 2 diabetes. J Transl Med 3:85.
- Salgueiro G, Beltrán LM, Torres RJ, Puig JG (2014) Fenofibrate increases serum creatinine in a patient with familial nephropathy associated to hyperuricemia. Nucleosides Nucleotides Nucleic Acids 33(4-6): 181-184.
- Balakumar P, Varatharajan R, Nyo YH, Renushia R, Raaginey D, et al (2014) Fenofibrate and dipyridamole treatments in low-doses either alone or in combination blunted the development of nephropathy in diabetic rats. Pharmacol Res 90:36-47.
- Tannock L [2018] Dyslipidemia in Chronic Kidney Disease. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com,
- Su SL, Lin C, Kao S, Wu CC, Lu KC, et al (2015) Risk factors and their interaction on chronic kidney disease: A multi-centre case control study in Taiwan. BMC Nephrol 16: 83.
- Ali SI, Alhusseini NF, Atteia HH, Idris RA, Hasan RA (2016). Renoprotective effect of a combination of garlic and telmisartan against ischemia/reperfusion-induced kidney injury in obese rats. Free Radic Res. 50(9): 966-86.
- Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, et al (2007) Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney International 72(3): 247–259.
- Zhang L, Wang F, Wang L, Wang W, Liu B, et al (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379(9818): 815–822.
- Apte UM, Limaye PB, Desaiah D, Bucci TJ, Warbritton A, et al (2003) Mechanisms of increased liver tissue repair and survival in diet-restricted rats treated with equitoxic doses of thioacetamide. Toxicol Sci 72(2): 272-282.
- Reynaert H, van Rossen E, Uyama N, Chatterjee N, Kumar U, et al (2007) Expression of somatostatin receptors in splanchnic blood vessels of normal and cirrhotic rats. Liver Int 27(6): 825-831.
- Murad HA, Gazzaz ZJ, Ali SS, Ibraheem MS (2017). Candesartan, rather than losartan, improves motor dysfunction in thioacetamide-induced chronic liver failure in rats. Braz J Med Biol Res. 50(11): e6665.
- Halici Z, Bilen H, Albayrak F, Uyanik A, Cetinkaya R, et al (2009) Does telmisartan prevent hepatic fibrosis in rats with alloxan-induced diabetes? Eur J Pharmacol 614: 146–152.
- Gross V, Schneider W, Schunck WH, Mervaala E, Luft FC (1999) Chronic effects of lovastatin and bezafibrate on cortical and medullary hemodynamics in deoxycorticosterone acetate-salt hypertensive mice. J Am Soc Nephrol 10(7): 1430-1439.
- Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3): 469-474.
- Buege J, Aust S (1978) Microsomal lipid peroxidation. Methods Enzymol 52: 302–310.
- Sastry K, Moudgal R, Mohan J, Tyagi J, Rao G (2002) Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal. Biochem 306: 79–82.
- VanGuilder HD, Vrana KE, Freeman WM (2008) Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques 44:619-626.
- Natarajan SK, Basivireddy J, Ramachandran A, Thomas S, Ramamoorthy P, et al. (2006) Renal damage in experimentally-induced cirrhosis in rats: Role of oxygen free radicals. Hepatology 43(6): 1248-1256.
- Ansil PN, Nitha A, Prabha SP, Wills PJ, Jazaira V, et al (2011) Protective effect of Amorphophallus campanulatus (Roxb.) Blume. tuber against thioacetamideinduced oxidative stress in rats. Asian Pac J Trop Med 4(11): 870-877.
- Begum Q, Noori S, Mahboob T (2011) Antioxidant effect of sodium selenite on thioacetamide-induced renal toxicity. Pakistan Journal of Biochemistry and Molecular Biology 44(1): 21–26.
- Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, et al (2008) Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int 73(12): 1374-1384.
- Nagothu KK, Bhatt R, Kaushal GP, Portilla D (2005) Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int 68(6): 2680-2693.
- Li H, Li M, Liu P, Wang Y, Zhang H, et al (2016) Telmisartan Ameliorates Nephropathy in Metabolic Syndrome by Reducing Leptin Release from Perirenal Adipose Tissue. Hypertension 68(2): 478-490.
- Narita Y, Ueda M, Uchimura K, Kakizoe Y, Miyasato Y, et al (2016) Combination therapy with renin-angiotensin-aldosterone system inhibitor telmisartan and serine protease inhibitor camostat mesilate provides further renoprotection in a rat chronic kidney disease model. J Pharmacol Sci 130(2): 110-116.






























