Assessment of Hepatoprotective Activity of Caralluma Tuberculata Stem Against Ccl4-Induced Liver Damage in Rabbits in Sherani Distruct of Balochistan
Sami Ullah Sherani, Javied Iqbal Amanullah Khan, Muhammad Ali Khan*, Nadeem Rashid, Mohammad Masood Tariq, Zia ud Din and Saadullah Jan
University of Baluchistan Quetta, Pakistan
Submission: January 05, 2019;Published: January 29, 2019
*Corresponding author: Muhammad Ali, University of Baluchistan Quetta, Pakistan
How to cite this article: Sami U S, Javied I A K, Muhammad A K, Nadeem R, Mohammad M T, Zia ud D, Saadullah J. Assessment of Hepatoprotective Activity of Caralluma Tuberculata Stem Against Ccl4-Induced Liver Damage in Rabbits in Sherani Distruct of Balochistan. J of Pharmacol & Clin Res. 2019; 6(5): 555697. DOI: 10.19080/JPCR.2019.06.555697
Abstract
Liver disease has become one of the serious health problems as it is exposed to many kinds of xenobiotic and therapeutic agents. Acute liver failure (ALF) is a potentially fatal complication of severe hepatic illness resulting from viral hepatitis or drug use. Some species of Caralluma are edible and form part of the folk medicine system of many countries. These are commonly used in traditional medicine as remedies to relive wide variety of illness and health problems. Silimyarin is the extract of silybum marianum, or milk thistle, and consists of seven flavonoglignans (silibinin, isosilibinin, silychristin, isosilychristin and silydianin) and a flavonoid (taxifolin). Caralluma species have shown anti-inflammatory, anti-nociceptive antidiabetics Hepatoprotective gastric mucosa protecting antimalarial antioxidant, anti-trypanosomal, appetite suppressant and cytotoxic activities. The present study aimed to evaluate the hepatoprotective effect of Methanolic Extract of Caralluma Tuberculata (MCCTE) in rabbits with acute liver injury induced by carbon tetrachloride at a single dose of 1.25 ml/kg b.w. as a mixture with olive oil. MCCTE was administered in doses of 500mg/kg b.w.) Via oral gavage by intragastric tube for 15 days. The SGOT, SGPT, ALP, total protein. The results of the rabbits treated with MCCTE were compared with that of standard drug showed a significant reduction (P< 0.05) in the hepatic enzymes levels, improvement of serum protein. These results suggested that MCCTE may be acting as a natural hepatoprotective agent that prevents hepatic injury induced by CCl4 and this may be due to its active phytoconstituents such as flavonoids which present in the plant.
Keywords: Silimyarin; Caralluma tuberculate; Acute liver failure; Hepatoprotective Activity; CCl4
Introduction
In the past decade, there has been a great increase in the use of complimentary treatments such as herbal remedies in the treatment of various diseases [1] Liver is the largest and most complex internal organ in the body, which plays an important role in maintenance of internal environment with the help of its multifarious and several functions [2].It is connected to inter mediatory metabolism of proteins, fats, and carbohydrates as well as in the synthesis of number of plasma proteins [3].such as albumin, fibrinogen and in the production of various enzymes, formation and excretion of bile [4] such as paracetamol, carbon tetrachloride, thioacetamide and isoniazid catabolize the radicals, bring on lipid peroxidation, damage the membranes of liver cells and organelles, cause the inflammation and necrosis of hepatocytes and leads to the liberation of cytosolic enzymes into the systemic transmission [5]. The most common disease of the liver is jaundice can be presented as yellow coloration of eye sclera, skin and mucous membrane due to increase amount of bilirubin in body, having prehepatic, hepatic or post-hepatic causes [6].
Plants and plant products are part of the vegetarian diet and several them exhibit medicinal properties. Silymarin is a standardized mixture of antioxidant flavonolignans (silybin and silibinin) extracted from the medicinal plant Silybum marianum [7] Silymarin is the extract of silybum marianum, or milk thistle, and consists of seven flavonoglignans (silibinin, isosilibinin, silychristin, isosilychristin and silydianin) and a flavonoid (taxifolin) [8]. Among these substances, silybin is mainly prevalent and has the most important biological effect. It makes up about 70% of the total composition of silymarin in the form of two diastereo-isomeric compounds: silybin a and silybin b [9].
Silymarin was proved to have a protective effect against experimental hepatotoxicity by regulating the actions of the ultrastructure of liver cells and improving the performance of hepatic enzymes and bile production [10].The purpose of this study was to evaluate the Hepatoprotective effect of the stem of C. Tuberculata against the CCL4 induced hepatotoxicity in rabbits (Figure 1).The local people has been using this plant since long for bitter tonic, febrifuge, stomachic and carminative useful in rheumatism internal wounds, diabetes, throat pain, fever, paralysis and joint pain ,weight loss, consumed as vegetable and for economical purposes. Furthermore, the other uses such as fresh plant is chewed, ear inflammation, Dysmenorrhea, abdominal pain, constipation and hepatitis B & C Peptic ulcer.

Materials and Methods
Selected plants material
Caralluma Tuberculata are edible and form part of the folk. This plant belonging to the family of Asclepiadaceae, which is also known as the milkweed family because many of its members contain milky latex Some species of Caralluma are edible and form part of the folk medicine system of many countries [11]. It is commonly used in traditional medicine as remedies to relive wide variety of illness and health problems [12].
Samples collection area
The Fresh plant was collected in April and May from various locations around the hill of village Ahmadedergah near to Tahkht-a- Suleiman district Sherani Balochistan, Pakistan (Figure 2). The collected samples authenticated by department of Pharmacognosy faculty of Pharmacy, University of Baluchistan, Quetta under the specimen’s voucher.

Preparation of extract
The fresh plants Caralluma Tuberculata were exposed to washing, then desired materials were left for 60 minutes after that the stem were chopped in small pieces and dehydrated at 25oC for 60 days under shade. The dried fragments were milled into fine powder [13]. Maceratedinmethanol at room temperature, occasional shaking as well as stirring done on alternate day for fifteen days followed filtration [14]. The extracts obtained were concentrated via Heidolph rotary serial number 519-00000-003, made of (Germany) evaporation at (45°C). Semi-solid (CMEs) was obtained, kept at 4°C until screened for further processing [15] (Figure 3).
Experimental animals
Healthy adult rabbits (1000-1300g) were obtained from the Center for Advanced studies in Vaccinology and Biotechnology (CASVAB), UoB. The animals were kept in a well-ventilated room and the animals were exposed to 12 hrs day and night cycle with a temperature between 20±3ºC. The animals were housed in large spacious, hygienic polypropylene cages during the course of the experimental period. The animals were fed with water and rat feed ad libitum, supplied by this institution. All the experiments were performed after obtaining prior approval of the Animal Ethics Committee (AEEC) of university of Balochistan, Quetta (Figure 4).

Drugs and reagents
Drugs and Chemicals Drugs and reagents were used include: Carbon Tetrachloride from ((Merck) standard Kits for assay of Aspartate transaminase (AST), Alanine transaminase (ALT), Alkaline phosphatase (ALP) activities in addition to, total protein, bilirubin, albumin levels were purchased from the local supplier. All other reagents and solvents were of highest purity and analytical grade [16].
Extract dose preparation
The dose was based on the body weight (mg/kg) and calculated through following formula
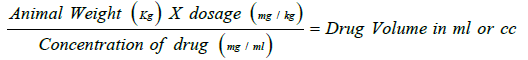
The Methanolic crude extracts (MCE) were dissolved and diluted with distilled water. Controlled animals were treated with an equal volume of saline solution of raw pieces [17].
Experimental design
The animals were distributed into 4 groups 5 rabbits in each group.
Control rats given physiological saline solution 10 mL/kg body wt, p. o for 14 days only.
Rabbits given silymarin (25 mg/kg body wt / p. o for 14 days only.
Rabbits given Caralluma Tuberculata (500 mg/kg body wt / p o) for 14 days only.
Rabbits given CCL4 + Caralluma Tuberculata (1.25 ml/ kg b.w. as a mixture with olive oil + 500 mg/kg body wt / p o) respectively on day 16th.
Rabbits given CCL4 + silymarin (25 mg/kg body wt / p. o for 14 days only on day 16th
At the end of the experimental period in 24h after last treatment the animals were killed by cervical decapitation. Blood was collected without anticoagulant for the separation of serum.
Biochemical analysis
Blood samples were taken into centrifuge tube with rubber caps labeled and centrifuged at 6000 rpm for 15-20 minutes. Serum biochemical parameter such as Transaminases (AST and ALT), AkP, GGT, Bilirubin and protein levels were estimated according to standard methods. The supernatant was stored in the freezer at -21 0C until analysis [18] (Figure 5).
Time period
Upon carrying out all the animal trials, the experiment was finished subsequently after 15 days the oral dose (30 to 40 min) of the material [19].
Administration route
The oral route was used for plant extract, standard drug and CCL4(Figure 6).


Statistical analysis
All statistical analysis, including the calculations of mean ± SE. the Student’s t-test, and the simple linear regression analysis, were carried out using SPSS and Graph Pad Prism programs. Significance was set at P< 0.05 level.
Results
Hepatoprotective test
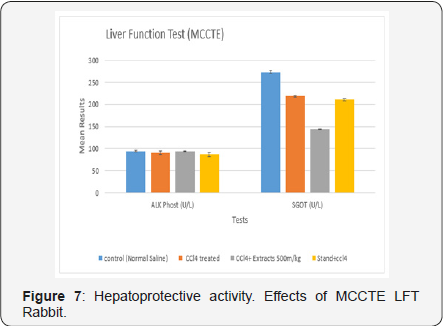
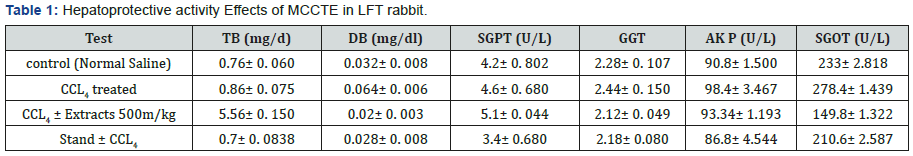
Liver function test: Findings in the current test of TB, DB, ALT, AKP, GGT and SGOT for the controlled group were, 0.76± 0.060, 0.032± 0.008 (mg/dL) 4.2± 0.802, 2.28± 0.10, 72.28±0.107, and 233± 2.818 (U/L), while, the same parameters were calculated in CCL4 treated group as 0.86± 0.075. 0.064±0.006 (mg/dL), 4.6± 0.680, 90.4± 3.467, 2.28± 0.107 and 278.4± 1.439 (U/L), respectively (Figure 7). Furthermore, the same parameters were found in the group treated with CCL4 and MCOLE 500mg, as 5.56± 0. 150, 0.02± 0. 003 (mg/dL) 5.1± 0. 044, 2.12± 0. 049, 93.34± 1.193and 149.8± 1.322, (U/L) [20]. Whereas, for the Standard drug (Silimyarin) and CCL4 treated group, values were 0.7± 0.0838, 0.028± 0.008, 3.4± 0.680 (mg/dL), 2.18± 0.080, 86.8± 4.544, 2.18± 0.0802 and 210.6± 2.587 (U/L), as shown in the (Table 1) [21].
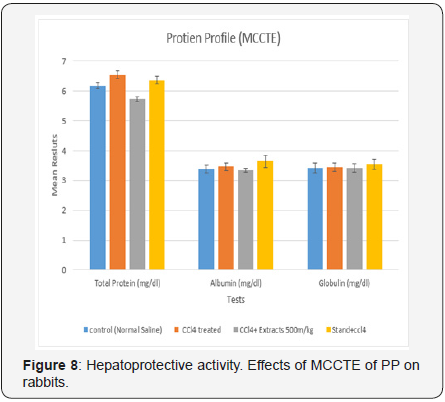
Protein profile (PP): The values for TP, Al, Gb, in the current study were 6.18± 0.097, 3.38± 01.132 and 3.42± 0.165 (g/dL), while, the values of the same parameters in the CCL4 treated group were,16.541± 01116, 6.54 ± 0.116 and16.54± 01.16 (g/ dL), whereas, the same parameters for the MCCTE 500m1g/kg and CCL4 treated group, 5.72± 0.080, 3.34± 0. 068 and 3.42± 0.143(g1/dL), respectively. Furthermore, these parameters for the standard drug ± CCL4 were calculated as 6.36± 0. 133, 3.64± 0.211 and 3.54± 0.163 as shown in the (Table 2).
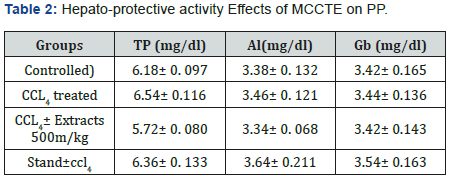
Values are the mean number of activities ± standard errors mean (n=5), (P<0.05) PP= Protein profile, TP = total protein, Al = Albumin, GB= Globulin (Figure 8).
Discussion
Phytochemical screening of Methanolic Crude C. tuberculata Extract (MCCTE) has revealed the presence of steroids, flavonoids, tannins, glycosides, saponins, carbohydrates and triterpenes. Phyto-constituents like flavonoids, Triterpenoids, saponins and alkaloids are known to possess Hepatoprotective activity [22]. MCCTE possesses a significant protective effect against hepatotoxicity induced by CCL4 which may be attributed to the individual or combined action of phytoconstituents present in plant [23]. The liver protective herbal drugs have been shown to contain a variety of chemical constituents like phenols, coumarins, ligands, essential oils, monoterpenes, carotenoids, glycosides, organic acids, lipids, xanthene [24].
The effect of CCL4 is generally observed after 24 h of its administration and then values slowly normalized. Hence the withdrawal of the blood for biochemical parameters should be carried out only after 24 h of CCL4 intoxication [25].The liver marker enzymes (AST, ALT and ALP) are largely used as most common biochemical markers to evaluate liver injury [26] because these enzymes are placed in cytoplasmic area of the cell and are released into circulation in case of cellular damage [27]. Thus, the activities of these enzymes in serum can reflect the severity of liver injury [28]. The elevated activities of these enzymes are indicative of cellular leakage and loss of the functional integrity of the cell membranes in liver which is always associated with hepato-necrosis [29].
In the present study CCL4 ± liquid paraffin administrated animals showed increased level of AST, SGOT, GAMA. GT, TP, Globulins. DB, TB, SGPT (ALT), but a decreased level of AKP and albumin, similarly, the standard drug (Silimyarin) showed defending property against CCL4 treated group [30]. Where as, close results were obtained by the MCCTE (500mg/kg) treated group against CCL4 during the present study [31]. Treatment with MCCTE+CCL4 significantly decreased levels of AST, ALT and ALP towards the normal value. These observations agree with the commonly accepted view that serum levels of transaminases return to normal due to stabilization of plasma membrane as well as repair of hepatic tissue damages caused by CCL4 [32].
Reduction in total protein content can be deemed as a useful index of severity of hepatocellular damage. In the present study, CCL4 intoxication reduced the serum total protein level which is attributed to the initial damage produced and localized in the endoplasmic reticulum. This indicated a decrease of cytochrome P-450 enzymes leading to its functional failure with a decline in protein synthesis and buildup of triglycerides leading to fatty liver [33]. Earlier studies exhibited that administration of CCL4 to various types of animal results in a rapid reduction in protein synthesis in the liver [34]. The treatment of MCCTE+CCL4 returned the total protein level which proposes the maintenance of endoplasmic reticulum leading to protein synthesis [35].
Conclusion
The present study produced convincing evidence that Methanolic Crude Extract (MCE) of Wild C. tuberculata has a Hepatoprotective effect against chemically induced CCL4.
References
- Yanardag Rosho, Ozsoy-Sacan O, Bolkent S, Orak H, Karabulut-Bulan O, et al. (2005) Protective effects of metformin treatment on the liver injury of streptozotocin-diabetic rats. Human & experimental toxicology 24(3): 129-135.
- Calixto JB (2000) Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Brazilian Journal of medical and Biological research 33(2): 179-189.
- Badman MK, Pissios P, Kennedy AR, Koukos G., Flier JS, Maratos Flier E (2007) Hepatic fibroblast growth factor 21st is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell metabolism 5(6): 426-437.
- Sherlock, Shiela , James Dooley (2008) Diseases of the liver and biliary system. John Wiley & Sons p. 832.
- Saleem M, Naseer F, Ahmad S, Nazish A, Bukhari RA, et al. (2014) Hepatoprotective Activity of Ethanol Extract of Conyza bonariensis against Paracetamol Induced Hepatotoxicity in Swiss Albino Mice. Amer J of Med and Biol Res 2(6): 124-127.
- Lone ZA, Lone Y, Khan SS, Wani AA, Reshi MI (2015) Hepatoprotective medicinal plants used by the Gond and Bhill tribals of District Raisen Madhya Pradesh, India. Journal of Medicinal Plants Research 9(12): 400-406.
- Wu JW, Lin LC, Tsai TH (2009) Drug-drug interactions of silymarin on the perspective of pharmacokinetics. J Ethnopharmacol 121(2): 185-193.
- Ahmed MA, Hamid AK, Tayawi HM (2018) Silymarin effect as an antioxidant to improve damages induced by CCl4 on some characteristics of male rats’ reproductive system. Tikrit Journal of Pure Science 23(2).
- Platzbecker U, Avvisati G, Cicconi L, Thiede C, Paoloni F, et al. (2017) Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. Journal of Clinical Oncology 35(6): 605-612.
- Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C (2005) Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. The Journal of nutritional biochemistry 16(10): 577-586.
- Hussain KB, Fontana RJ, Moyer CA, Su GL, Sneed Pee N, et al. (2001) Comorbid illness is an important determinant of health-related quality of life in patients with chronic hepatitis C. The American journal of gastroenterology 96(9): 2737-2744.
- Barnes PM, Powell Griner E, Mc Fann K, Nahin RL (2004) Complementary and alternative medicine use among adults: United States 2002 In Seminars in integrative medicine. WB Saunders 343: (1-19).
- Rehman R., Chaudhary MF, Khawar KM, Lu G, Mannan A, et al. (2014) In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia 69(3): 341-349.
- Sadasivam S, A Manickam (1996) Biochemical methods. New age international 2: 124-126.
- Haddarah A, Bassal A, Ismail A, Gaiani C, Ioannou I, et al. (2014) The structural characteristics and rheological properties of Lebanese locust bean gum. Journal of Food Engineering 120: 204-214.
- RaufA, Hadda TB, Mubarak MS, Patel S (2015) Quantitative ethnobotanical survey of medicinal flora thriving in Malakand Pass Hills, Khyber Pakhtunkhwa, Pakistan. Journal of ethnopharmacology 169: 335-346.
- Nair AB., Kumria R, Al-Dhubiab BE, Attimarad M, Harsha S (2016) Development of transdermal delivery system of vildagliptin and its comparison with oral therapy. Indian J Pharm Educ Res 50(1): 130-137.
- Baxla SL (2012) Evaluation of Efficacy of Withania somnifera AND Curcuma longa Against Lead Induced Sub-Acute Toxicity in Albino Rats. (Doctoral dissertation, Birsa Agricultural University, Kanke, Ranchi, Jharkhand.
- Lee WM (2003) Drug-induced hepatotoxicity. New England Journal of Medicine 349(5): 474-485.
- Martín R, Carvalho Tavares J, Hernández M, Arnes M, Ruiz-Gutierrez V, et al. (2010) Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: a potential therapeutic role. Biochemical pharmacology 79(2): 198-208.
- Rathi A, Srivastava AK, Shirwaikar A, Rawat AKS, Mehrotra S, et al. (2008) Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine 15(6-7): 470-477.
- Sailaja R, OH Setty (2006) Protective effect of Phyllanthus fraternus against allyl alcohol-induced oxidative stress in liver mitochondria. Journal of Ethnopharmacology 105(1-2): 201-209.
- Suresh Kumar SV, Sujatha C, Syamala J, Nagasudha B, Mishra SH (2007) Hepatoprotective activity of extracts from induced toxicity in rats. Phcog Mag 3(11): 187-191.
- Adhvaryu MR, Reddy N, Parabia MH (2007) Effects of four Indian Medicinal herbs on isoniazid and pyrazinamide induced hepatic injury and immunosuppression in guinea pigs. World Journal of Gasteroenterlogy 13: 3199-3205.
- Sreelatha S, Padma PR (2009) Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant foods for human nutrition 64(4): 303-311.
- Kozer E, Evans S, Barr J, Greenberg R, Soriano I, et al. (2003) Glutathione, glutathione-dependent enzymes and antioxidant status in erythrocytes from children treated with high-dose paracetamol. Br J Clin Pharmacol 55(3): 234-240.
- Girish C, Koner BC, Jayanthi S, Rao KR, Rajesh B,Pradhan SC (2009) Hepatoprotective activity of six poly herbal formulations in paracetamol-induced liver toxicity in mice.Indian J Med Res 129: 569-578.
- Brent JA, Rumack BH (1993) Role of free radicals in toxic hepatic injury II. Clin Toxicol 31(1): 173-196.
- Naik SR, Panda VS (2008) Hepatoprotective effect of Ginkgo select phytosome in rifampicin induced liver injury in rats: evidence of antioxidant activity. Fitoterapia 79(6): 439-445.
- Verma, Nitin, RL Khosa (2010)"Hepatoprotective activity of leaves of Zanthoxylum armatum DC in CCl 4 induced hepatotoxicity in rats. Indian J Biochem Biophys 47(2): 124-127.
- Al Mehdar AA, El Denshary ES, Abdel Wahhab MA (2012) Alpha lipoic acid and alpha-tocopherol counteract the oxidative stress and liver damage in rats sub-chronically treated with khat (Catha edulis) Extract. Global J of Pharmacology 6(2): 94-105.
- Rajesh MG, Latha MS (2004) Preliminary evaluations of the antihepatotoxic effect of Kamilari, a polyherbal formulation. J Ethnopharmacol 91(1): 99-104.
- Muthulingam M (2002) Studies on the curative efficacy of Astercantha longifolia on carbon tetrachloride induced hepatotoxicity in rats. Thesis, Annamalai University, Sodhganga.
- (2007) Pergulari adaemia Forsk against Carbon tetrachloride induced toxicity in rats. Phcog Mag,India.
- Ranawat L, Bhatt J, Patel J (2010) Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4 induced hepatic damage in rats. J Ethnopharmacol 127(3): 777-780.






























