Evaluation of Hepato-Protective Activity of B. Lycium Methenolic Crude Extracts Collected from Distric Sherani, Balochistan
Sami Ullah Sherani, Javied Iqbal, Amanullah Khan, Muhammad Ali Khan*, Nadeem Rashid, Mohammad Masood Tariq, Zia ud Din and Saadullah Jan
University of Baluchistan Quetta, Pakistan
Submission: January 05, 2019;Published: January 25, 2019
*Corresponding author: Muhammad Ali, University of Baluchistan Quetta, Pakistan
How to cite this article: Sami U S, Javied I, Amanullah K, Muhammad A K, Nadeem R, et al. Evaluation of Hepato-Protective Activity of B. Lycium Methenolic Crude Extracts Collected from Distric Sherani, Balochistan. J of Pharmacol & Clin Res. 2019; 6(5): 555696. DOI: 10.19080/JPCR.2019.06.555696
Abstract
Berberis lycium, which is commonly called as Indian barberry (English) and Kashmal or Ishkeen in Urdu, is a spiky plant which is the member of the genus Berberis of family Berberidaceae. B. lycium also includes anti-hepatotoxicity effect, when was mixed with G. aparine and P. integerrima and was tested in rats that were treated with carbon tetra chloride; the results revealed that the combination of these three medicinal plants encompasses anti hepatotoxicity effects. B. lycium (root) sample was collected from the hill of village of Ahmadedergah near to Tahkhta Suleiman District Sherani Baluchistan, Pakistan. The collected samples were identified by Pharmacognosy, Department faculty of Pharmacy University of Baluchistan, Quetta. Adult healthy rabbits (male) having weights approximately 950g-1300g, were kept in animals house at CASVAB, UoB.
Sample serum, within 3 hours after collection, was analyzed for certain biochemical parameters (Alkaline Phosphatase (ALP), Alanine Aminotransferase (ALT), Gamma Glutamyl Transpeptidase (γ-GT), Aspartate aminotransferase (AST), and total Proteins like Bilirubin, Albumin and Globulin) through automatic analyzer (Merck) at 37° C through standard reagent kits. The obtained values for LFT in the current portion of controlled group were, 0.76± 0.060, 0.032±0.008 (mg/dL), 4.2±0.802, 93.8±1.500, 2.28±0.107 and 273±2.818 (U/L) for TB, DB, ALT, AKP, GGT, SGOT and SGPT (AST) levels. Whereas, the group treated with CCl4 these parameters were, 0.86±0.075, 0.064+0.006 (mg/dL), 4.6±0.680, 2.44±0.150, 90.4±3.467 and 218.4±1.439 (U/L) level. While, the group treated with CCl4 and B. lycium 500mg, the above parameters were calculated as 0.92±0.086, 0.442±0.228 (mg/dL), 4.4±0.601, 93.2±1.244, 2.62±0.097 and 221.6±1.540 (U/L), respectively.
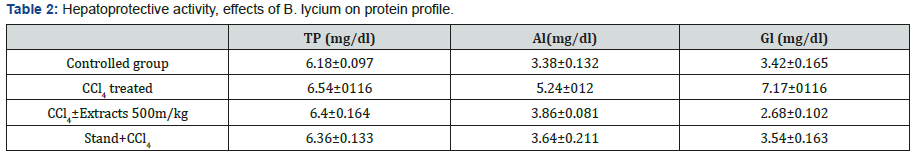
In controlled group the values for Total Proteins, Albumin and Globulin, were 6.18±0.097, 3.38±0.132 and 3.42±0.165 (g/dL), while, the values for the same parameters in CCl4 treated group were, 6.54±0116, 5.24±012 and 7.17±0116 (g/dL) respectively. Whereas, the same parameters were calculated in the group treated with B. Lycium 500mg/kg and CCl4, the resulted values were 6.4±0.164, 3.86±0.081and 2.68±0.102 (g/dL), respectively.
Keywords: Berberis lyceum; Sherani; Hepatoprotective activity; Balochistan; LFT; PP
Introduction
In Pakistan, many native plants are utilized in herbal medicine to treat diseases and injuries. Such plants often show a broad spectrum of biologic and pharmacologic activities, for example, they are able to reduce inflammation, having antibacterial and antifungal properties Cowan MM [1]. The root, bark, seed and fruit extracts of the medicinal plants are used in syrups and infusions, traditionally [2]. Berberis lycium, which is commonly called as Indian barberry (English) and Kashmal or Ishkeen in Urdu, is a spiky plant which is the member of the genus Berberis of family Berberidaceae [3]. It is found in the moderate and semitropical Asian, European and American divisions [4]. In Pakistan it is extensively distributed in Baluchistan, KPK and Punjab provinces, especially in northerly regions, Swat and Azad Kashmir at elevation of 900 to 2900m [5] B. lycium is a vertical flowering bush plant that increases to a length of 3-4 meters, having a solid timber stem and is enclosed in a slight fragile bark. The branches of B. lycium are light white to grayish and have thorns alternatively fixed on them [6]. Root bark could be approximately 3 mm solid, from the outside fractured and inside smooth [7] B. lycium is extensively utilized for the treatment UTI, swelling of spleen, stomach and intestinal ulcer and liver diseases.
Local population utilizes the powdered form of dried root bark after combining with dissolved animal fat for bone fractures as a bandage. Shoots of the plant are employed for the abdomen pain, jaundice and loose bowels [8]. The bark of the plant has wound healing activity [9] (Figure 1). B. lycium also includes anti-hepatotoxicity effect, when was mixed with G. aparine and P. integerrima and was tested in rats that were treated with carbon tetra chloride; the results revealed that the combination of these three medicinal plants encompasses anti hepatotoxicity effects [10]. To estimate Hepatoprotective effect of B. lycium, crude powder and Methanolic extract of plant were used. CCl4 was given to the rabbits to induce hepatotoxicity [11]. Results showed that plant considerably decreased the elevated levels of serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase and alkaline phosphatase enzymes in hepatotoxic rabbits [4]. Herbo-med, Kolkata in another study six poly herbal formulations including Livokin and B. lycium in mice. This formulation showed Hepatoprotective effect in paracetamol stimulated hepatotoxic mice [7]. The present study was therefore designed to evaluate the in vitro heptoproductivity effect of B. lycium.

Materials and Methods
Berberis Lycium (root) sample was collected from the hill of village of Ahmadedergah near to Tahkhta Suleiman District Sherani Balochistan, Pakistan. The collected samples were identified by Pharmacognosy, Department faculty of Pharmacy University of Baluchistan, Quetta under the specimen’s voucher (No. S-243) (Figure 2).

After the collection of the plant roots following procedure was adapted;
a. The fresh plant of B. lycium roots were exposed to washing and chopping [12].
b. Then desired materials were left for 60 minutes (Lust, 2014).
c. After that the herb was cut into small pieces.
d. Dehydrated is shed at 25°C for 30 days.
e. The dried fragments were milled into fine powder.
f. Macerated in methanol at room temperature, occasional shaking as well as stirring done on alternate day for fifteen days followed filtration.
g. The extracts obtained were concentrated via Hieroglyph rotary serial number 519-00000-003, made of (Germany) evaporation at (45°C).
h. Semi-solid (CMEs) was obtained, kept at 4°C until screened for further processing [13].
Adult healthy rabbits (male) having weights approximately 950g-1300g, were kept in animals house at CASVAB, UoB. The selected animals were kept in metal cage and feed with clean water and food throughout the study period under standard protocol of relative humidity, [14] temperature (22 ± 1ºC) and 12hrs light/dark cycles and adapted for 1 week earlier the test in the research Laboratory of CASVAB. Before conducting the research, the procedure for research was approved by the Animal Ethics Committee (AEEC) of university of Balochistan, Quetta. Silimyarin (DTL) liquid paraffin and Media of analytical grade were used in in the experiments [15].
The dose was based on the body weight (mg/kg) and calculated through following formula
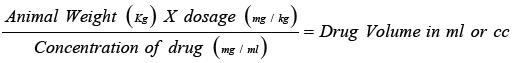
The Methanolic crude extracts (MCE) were dissolved and diluted with distilled water. Controlled animals were treated with an equal volume of saline solution [16]. The oral route was used for plant extract while for the standard and control oral and intraperitoneal (i.p) and subcutaneously route was used. a. Rabbits were adapted for seven days before experiment and housed in standard temperature humidity and light conditions with 12-hour light/ darkness cycle (Good Laboratory) were used [17].
b. Rabbits weighing 1000-1400 gm in the control group (group 1), NaCl 0.9% (1ml / Kg)
c. The treated group was given CCl4 (Carbon tetrachloride) (Group II).
d. Plant extract (MCE) dose 500mg/kg/day was given to Group III [18].
e. Standard drug (Silimyarin) of dose 100 mg/kg/day administered to Group IV. (15 days)
f. On the sixteenth day the Groups 2.3,4 were administered CCl4, with 1:1, 1.5ml/Kg liquid paraffin afterward 30 min of the 2nd dosage of vehicle [19].
g. Group IV was treated with Silimyarin 100 μg/kg bw weight group along with CCl4 [20].
h. Group III were treated with MCE with the concentration of 500 mg/kg body weight, respectively with CCl4 [21].
i. After 36 hours of last dose, 5cc blood were taken in Eppendorf via cardiac puncture technique to obtain the serum [22]. The samples were kept in centrifuge machine up to 6000 rpm for ten minutes.
j. Sample serum, within 3 hours after collection, was analyzed for certain biochemical parameters (Alkaline phosphatase (ALP), Alanine aminotransferase (ALT) [23], Gamma glutamyl transpeptidase (γ-GT), Aspartate aminotransferase (AST), and total Proteins like Bilirubin, Albumin and Globulin) through automatic analyzer (Merck) at 37oC through standard reagent kits [24].
Results
Liver Function Test (LFT)
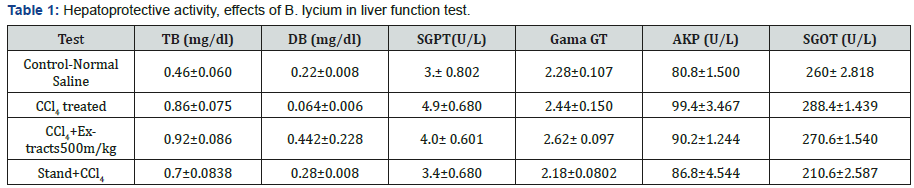
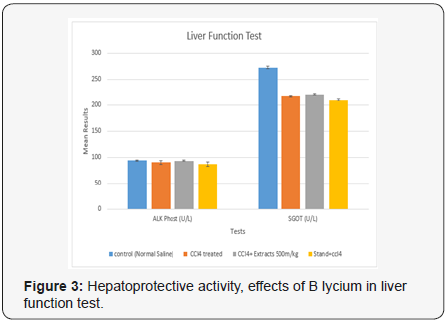
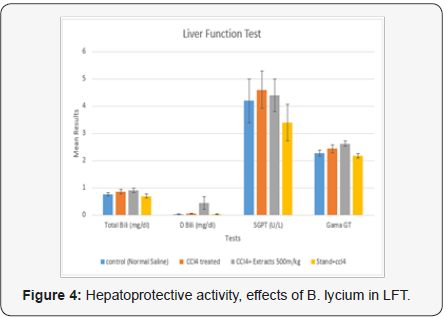
The obtained values for LFT in the current portion of controlled group were, 0.76± 0.060, 0.032±0.008 (mg/dL), 4.2±0.802, 93.8±1.500, 2.28±0.107 and 273±2.818 (U/L) for TB, DB, ALT, AKP, GGT, SGOT and SGPT(AST) levels. Whereas, the group treated with CCl4 these parameters were, 0.86±0.075, 0.064+0.006 (mg/ dL), 4.6±0.680, 2.44±0.150, 90.4±3.467 and 218.4±1.439 (U/L) level. While, the group treated with CCl4 and B. lycium 500mg, the above parameters were calculated as 0.92±0.086, 0.442±0.228 (mg/dL), 4.4±0.601, 93.2±1.244, 2.62±0.097 and 221.6±1.540 (U/L), respectively. Silimyarin, was used as a standard drug treated with CCl4 and the values for TB, DB, ALT, AKP, GGT, SGOT and SGPT were, 0.7±0.0838, 0.028±0.008 (mg/dL) 3.4±0.680, 86.8±4.544, 2.18±0.0802 and 210.6±2.587 (U/L), respectively (Table 1) (Figures 3 & 4).
Protein Profile
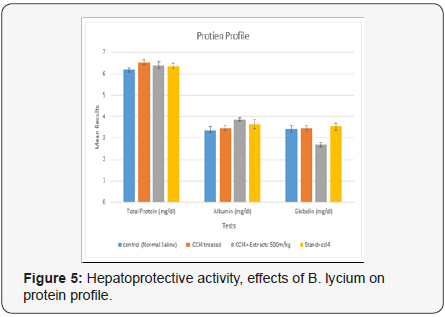
In controlled group the values for Total Proteins, Albumin and Globulin, were 6.18±0.097, 3.38±0.132 and 3.42±0.165 (g/ dL), while, the values for the same parameters in CCl4 treated group were [25], 6.54±0116, 5.24±012 and 7.17±0116 (g/dL) respectively. Whereas, the same parameters were calculated in the group treated with B. Lycium [26]500mg/kg and CCl4, the resulted values were 6.4±0.164, 3.86±0.081and 2.68±0.102 (g/ dL), respectively (Table 2) (Figure 5).
Discussion
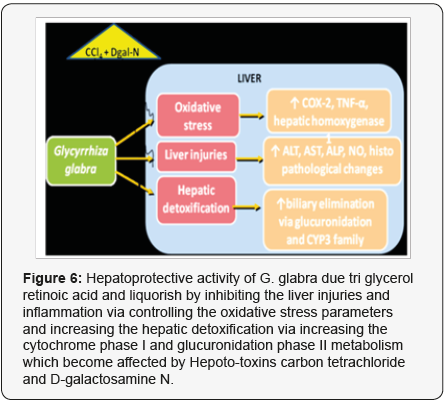
Liver is the main organ of metabolism and energy production. It regulates systemic lipid homeostasis, which is involved in the redistribution of lipoproteins, triacylglycerol for storage and utilization by peripheral tissues [22]. Several plant extracts have been examined for use in a wide variety of liver disorders, G. pentaphylla protects membrane integrity in mice hepatocytes [19]. S. marianum is a chemo-preventive agent that shows antitumor activity against human tumors in rodents (Agarwal et al., 2006). Similarly, several cases of hepatotoxic side effects of green tea have been reported in rats [19] (Figure 6). During the present study all the treated groups of the tested animals (rabbits) were given CCl4 according to the recommended doses and evaluated serum TB, DB, Alanine aminotransferase (ALT), Alkaline phosphatase (ALP)., Gamma glut-amyl transferees (GGT), SGOT and Aspartate aminotransferase (AST or SGPT) by Liver function test (LFT), furthermore, total protein (TP), Albumin (Al), Globulin (Gb) were calculated through Protein Profile test (PFT) [17].
It is revealed from the results of this study that the Methanic Crude Extracts (MCE) of B. lyceum at the dose of 500mg/kg body weight decreased serum TB, DB, ALT, AKP, GGT, SGOT and SGPT (AST) and TP, Al and Gb in the tested animals (rabbits) [27]. Higher activities of these enzymes in serum have been found in response to oxidative stress induced CCl4. This was also evidenced from the downturn in levels of marker enzymes of test groups compared with the toxic control group and pure drug [28]. The improvement in the enzyme activity was due to sustained and targeted action of Silimyarin and MCE on the hepatocytes due to their pronounced antioxidant effect may have diminished the release of SGOT, SGPT and ALP enzymes from the liver cells, [29] thereby eliciting Hepatoprotective activity [30] .The findings of the present study also showed antioxidant properties, which are comparable to some previous reported [31] studies of other plant extracts, having Hepatoprotective activity [32], B. lyceum showed strong free radical scavenging activity when extracted [33] by methanol [9]. The results of this study showed that after administration of MCE the activities [34] of the serum marker enzymes were returned to near to the standard drug (Silimyarin) [35].
Conclusion
Metholanic Crude Extracts of root of B. lycium showed remarkable Hepatoprotective activity in the experimental rabbits and the results were almost near to those of the Silimyarin drug, which was used as a standard drug during the study. Furthermore, the hepatotoxicity due to CCl4 can be reduced up to safe level through the usage of this plant extract. These results have showed some light on the clinical therapeutic potential of these MCE of B. lyceum against hepatotoxic agents. More studies should be performed similar to this research in order to specify the main phy to compounds present in this plant which may be responsible for Hepatoprotective activity.
References
- Cowan MM (1999) Plant products as antimicrobial agents. Clinical microbiology reviews p. 564-582.
- Imtiaz UH, Manzoor H (2003) Medicinal plants of Mansehra. Hamdard medicus 36: 69.
- Sabir S (2013) Phytochemical and antioxidant studies of Berberis lycium. Pakistan journal of pharmaceutical sciences 26(6): 1165-1172.
- Jabeen N, Saleem A, Anwaar S, Hussain Z (2015) Berberis lycium Royle (Royle, 1837): A threatened medicinal plant and Its biological activities. EC Agri culture 1(2): 100-108.
- Dhar S, sharma YP, Wakhlu AK (2012) In vitro plant regeneration system for Berberis lycium using cotyledonary node explants. Journal of Tropical Medicinal Plants 13(1): 51-55.
- Ahmad M, Alamgeer, Sharif T (2009) A potential adjunct to Insulin: Berberis lycium Royle. Diabetologia Croatica 38(1): 13-18.
- Chauhan, NS (1999) Medicinal and aromatic plants of Himachal Pradesh. Indus publishing, India.
- Beers SJ (2012) Jamu: the ancient Indonesian art of herbal healing. Tuttle Publishing, USA.
- Asif A, Kakub G, Mehmood S, Khunum R, Gulfraz M, et al. (2007) Wound healing activity of root extracts of Berberis lycium Royle in rats. Phytotherapy Research 21(6): 589-591.
- Thounaojam MC, Jadeja RN, Valodkar M, Nagar PS, Devkar RV, et al. (2011) Oxidative stress induced apoptosis of human lung carcinoma (A549) cells by a novel copper nanorod formulation. Food and chemical toxicology 49(11): 2990-2996.
- Ahmad M (2008) Hepatoprotective effect of Berberis lycium (Royle) in hepatotoxic rabbits. Gomal University journal of research pp. 24.
- Joseph B, Jini D (2011) Insight into the hypoglycaemic effect of traditional Indian herbs used in the treatment of diabetes. Research Journal of Medicinal Plant 5(4): 352-376.
- Yang Z, Zhai W (2010) Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innovative Food Science & Emerging Technologies 11(1): 169-176.
- Potdar D (2012) Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 83(5): 817-830.
- Zovko K, Kremer D, Karlovic K, Kosalic L (2010) Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food and chemical toxicology 48(8-9): 2176-2180.
- Shamsa F, Ahmadiani A, KhosroKhavar R (1999) Antithistaminic and anticholinergic activity of Berbery fruit (Berberis vulgaris) in the guinea pig ileum. Journal of Ethnopharmacology 64(2): 161-166.
- Amin A, Hamza AA (2005) Oxidative stress mediates drug-induced hepatotoxicity in rats: A possible role of DNA fragmentation. Toxicology 208(3): 367-375.
- Ivanovska N, Philipov S (1996) Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. International journal of Immunopharmacology 18(10): 553-561.
- Okamura H, Akio M, Yasuko Y, Misturu N (1993) Antioxidant activity of tannins and flavonoids in Eucalyptus rostrata. Phytochemistry 33(3): 557-561.
- Royle JF (1834) On the Lycium of Dioscorides. Transactions of the Linnean Society of London 17(1): 83-94.
- Kim YJ, Park T (2008) Genes are differentially expressed in the epididymal fat of rats rendered obese by a high fat diet. Nutr Res 28(6): 414-422.
- Demori I., Voci A, Fugassa E., Burlando B (2006) Combined effects of high-fat diet and ethanol induce oxidative stress in rat liver. Alcohol 40(3): 185-191.
- Lee CH, Olson P, Evans RM (2003) Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology, 144(6): 2201-2207.
- AgyareC, Asase A, Lechtenberg M, Niehues M, Deters A, et al . (2009) An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi Atwima Kwanwoma area Ghana. Journal of Ethnopharmacology 125(3): 393-403.
- Abere TA, Okoto PE, Agoreyo FO (2010) Antidiarrhoea and toxicological evaluation of the leaf extract of Dissotis rotundifolia triana (Melastomataceae). BMC complementary and alternative medicine 10(1): 71.
- Yang JJ, Li GL, Liu HR, Ren BB (2003) Effect of eveningrose oil on activities of oxygen free radical scaverging-related enzymes and hepatic morphosis in rats on high lipid diet. J NingXia Med Coll 25: 244-246.
- Yang JS, Lee SJ, Park HW, Cha YS (2006) Effect of genistein with carnitine administration on lipid parameters and obesity in C57Bl/6J mice fed a high fat diet. J Med Food 9(4): 459-467.
- Eisenberg DM, Kessler RC, Foster C, Norlock, FE, Calkins DR, et al. (1993) Unconventional medicine in the United States prevalence, costs, and patterns of use. New England Journal of Medicine 328(4): 246-252.
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, et al. (1998) Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. Jama 280(18): 1569-1575.
- Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, et al. (2002) Plants and human health in the twenty-first century. TRENDS in Biotechnology 20(12): 522-531.
- Abere TA, Okoto PE, Agoreyo FO (2010) Antidiarrhea and toxicological evaluation of the leaf extract of Dissotis rotundifolia triana (Melastomataceae). BMC complementary and alternative medicine 10(1): 71.
- Myagmar BE, Shinno E, Ichiba T, Aniya Y (2004) Antioxidant activity of medicinal herb Rhodococcum vitisidaea on galactosamine-induced liver injury in rats. Phytomedicine 11(5): 416-423.
- Paul, Daizy (2010) Studies on the Medicinal Flora of Amritsar District.
- Jafri SMH. Berberidaceae. Flora of Pakistan.
- Rong XL, Kim MS, Su N, Wen SP, Matsuo Y, et al. (2008) An aqueous extract of Salacia oblonga root, a herb-derived peroxisome proliferator-activated receptor alpha activator, by oral gavage over 28 days induces gender dependent hepatic hypertrophy in rats. Food Chem. Toxicol 46(6): 2165-2172.






























