APBVariation in the Functional Properties of Barringtonia Asiatica Extract on Selected Pathogens
Isaac John Umaru1,2*, Fasihuddin ABadruddin1, Hauwa AUmaru3, Ezeonu Chukwuma Stephen2 and Ojochenemi Yakubu
1Faculty of Resource Science and Technology, University of Malaysia Sarawak, Kuching, Kota-Samarahan, Malaysia
2Department of Biochemistry, Federal University Wukari Taraba State, Nigeria
3Department of biochemistry, ModiboAdama University of Technology Yola Adamawa state, Nigeria
Submission: August 24, 2018; Published: September 10, 2018
*Corresponding author: Isaac John Umaru,Faculty of Resource Science and Technology, University of Malaysia Sarawak, Kuching, Kota-Samarahan Malaysia,Department of Biochemistry Federal university Wukari Taraba State
How to cite this article: Isaac John Umaru, Fasihuddin ABadruddin, Hauwa AUmaru, Ezeonu Chukwuma Stephen, Ojochenemi Yakubu. APBVariation in the Functional Properties of Barringtonia Asiatica Extract on Selected Pathogens. J of Pharmacol & Clin Res. 2018; 6(3): 555686. DOI: 10.19080/JPCR.2018.06.555686
Abstract
Objective: The aim of this study is to look at the influence of different concentration from different solvent extract of Barringtonia asiatica on selected pathogens.
Material and Methods: Barringtonia asiatica extracts were evaluated for their functional potential antimicrobial properties. The leaves of the plant were extracted with n-hexane, dichloromethane, ethyl acetate, chloroform and methanol and then vaporized to give respective extracts. Antimicrobial activity against Escherichia coli, salmonella typhi, staphylococcus aureus and Klebsielia pneumonia, were determined by a disc diffusion method. The optical density of the broth using UV mini spectrophotometer and zone of inhibition by the crude extract were determined.
Results: The influence of chloroform and methanol crude extract of Barringtonia asiatica tested for its biological activity indicated high potential activity on all the pathogen despite their variation in extract concentration, the rate of inhibition is slightly high in all methanol concentration when compared to the chloroform extract concentration with increase in concentration in all the pathogen.
Conclusion: The present results showed the impact of concentration variation and the potential of the medicinal plant used by traditional herbal medical practitioners as natural antimicrobial agents. It is possible that the extracts contain compounds with potential biological activity that can be used to treat bacterial infections. Thus, can be further used to determine the bioactive products that may provide as leads in the development of new drugs.
Keywords: Barringtonia Asiatica; Pathogens;Chloroform; Methanol
Introduction
Medicinal plants have been associated with the prevention of degenerative diseases such as cancer and cardiovascular diseases. The presence of wide range of phytochemicals such as phenolics, thiols, carotenoids, anthocyanins and tocopherol have been suggested to exert chemo-preventive and cardio protective effects as well as protecting the human body against oxidative damage by free radicals [1]. Natural phytochemicals derived from fruits, vegetables and herbs have been reported to possess a wide range of biological effects, including antioxidant, antimicrobial and anti-inflammatory actions [2]. Most of this bioactive compound have been reported to demonstrate antibacterial, antiviral, anticarcinogenic, anti-inflammatory and vasiodilatory actions [3,4].
The genus Barringtonia is in the family Lecythidaceae in the major group Angiosperms (Flowering plants), Among which Barringtonia; asiatica (Linn.) Gaetrn is one natural plant with a lot traditional medicinal potential, it is a Malaysian Mangroves locally known as Putat Kampung Gajah [5], itis an evergreen tree found in East Africa, South East Asia (including Malaysia) and Pacific Islands. This plant is a small tree capable ofreaching 20m or more with leaves tufted at the ends of stout twigs and it is common in the moist low country, especially near the shores of back waters, lakes, rivers and the banks of paddy fields.
The leaves are used as vegetable as food, Herb, Spice, Backaches, sore joints, Rheumatism, Hernia, Diarrhea and to Poisoning the fishes for a cache [6]. Ethno medical survey has shown that the seeds of Barringtonia asiaticaare traditionally used to expelled intestinal worm, dried nut treats coughs, Ring worm, influenza, sore throat and bronchitis, Poisoning the fishes [6,7]. While the stem-bark are used as medicinal for Back-aches, stomach ache, tumors, epilepsy, constipation, tuberculosis and sore joints, Rheumatism, Poisoning the fishes also [6-10]. However, the fruits are used for food, treat swollen spleen after an attack of malaria, Poisoning the fishes, Treat hernia, cysts, goiter, tumor, boils, and all kinds of lumps or bumps [7,9,11]. The plant is expected to contain a saponin compound and saponin is included as a natural compound with large molecule mass and value [1,2,7,8]. Tenor et al. [12] reported the presence of saponnin which are potential to be the bio-pesticide and its work mechanism. Thus, literature survey revealed that there are little or no scientific studies carried out regarding antibacterial activity of the leaves of Barringtonia asiatica. Hence, the present study is focused to evaluate the antibacterial potentials of chloroform and methanol extracts of the leaf of Barringtonia asiatica.
Material and Methods
Chemicals
All chemicals used in this investigation were of analytical grade and were obtained from SIGMA. Standard antibacterial agent (30μg) tetracycline, antimicrobial susceptibility test discs and Nutrient agar (CM0003) were obtained from Oxoid Ltd, Wade Road, Basingstoke, Hants, RG2 8PW, UK.
Sample Collection
Barringtonia asiatica leaves: freshly leaves were obtained from Kampung Merekan at the bank of Merekan river in Sarawak Malaysia was authenticated by Prof Fasihuddin Ahmed Badruddin and Prof ZainiAssim and was deposited in the Natural Product Laboratory Universiti Malaysia Sarawak. The plant was dried under room temperature.
Preparation of Samples
The sample preparation as reported by Umaru et al. [13], Fresh leaves of the plant Barringtonia asiaticawere washed with distilled water to remove the soil and dust particles, they are thoroughly air dried and powdered using laboratory grinder machine (FGR-350, Quest Scientific). Extraction using hexane by placing 150g of the powdered samples into an Erlenmeyer flask and hexane three times the weight of the extracts was added, the solution was covered and shaken at an interval of an hour and then allowed at room temperature to stand for 7days. The mixture was then filtered using Whatman filter paper No.4 the residue was re-extracted with fresh hexane for another 72hrs and filtered. Both extracts were combined and concentrated with a rotary evaporator (HeidolphLaborota 4000 efficient) under reduced pressure toobtain the hexane crude extract. The residues were re-extracted using a similar procedure with dichloromethane (CH2CLl2), followed by ethyl acetate (C2H5COOH), chloroform (CHCL3), and methanol (MeOH) to obtain dichloromethane, ethyl acetate, chloroform and methanol crude extracts, respectively. The dry weight and yield of each crude extracts were determined. It was then stored under a frozen condition until required.
Antimicrobial Assay
Antimicrobial assays were conducted using the agar well disk diffusion method, Nutrient agar was used as media.
Preparation of Agar Plates
Preparation of agar plates was performed based on the method described by Umaru et al. [13]. Nutrient agar was prepared according to manufacturer’s instruction with 14g of dried agar dissolved in 500 ml distilled water. The agar solution was heated until boiling followed by sterilization in autoclave at 121°C. The agar solution was then poured into a sterile petri plate and allowed to cool down and forming a gel. The plate was divided into eight sections by making a line marking on the outside surface of the plate. The eight sections were for each test samples namely 25ppm, 50 ppm, 100 ppm, 250 ppm, 500 ppm and 1000 ppm samples, tetracycline 30μg (positive control) and methanol (negative control). The plate was sealed using parafilm and keep chilled at 4°C upon bacteria inoculation.
Preparation of Bacteria Broth
Several selected bacteria were used to evaluate the antibacterial activities of the crude extracts of Barringtonia asiatica. Escherichia coli,Salmonella typhi, Staphylococcus aureus and Klebsielia pneumonia were obtained from the stock culture provided by Virology Laboratory, University Malaysia Sarawak. The nutrient broth was prepared according to manufacturer’s instruction, with 2.6g of the dried broth dissolved in 200ml distilled water followed by sterilization in an autoclave at 121°C. The bacteria were sub-cultured in a 10 ml of broth, each in a universal glass bottle for 16hrs inside an incubator equipped with a shaker at 37°C[14]. After 16hrs incubation, turbidity (optical density/OD) of the bacterial broth was measured by using UV mini spectrophotometer (model 1240 of Shimadzu brand), comparable to that of nutrient broth standard tube for further use [15]. The measurement was performed at wavelength 575 nm and the bacterial broth was ready to be used when its turbidity is between OD 0.6 to 0.9. The nutrient broth was used to adjust the turbidity until the desired value was obtained.
Plate Inoculation
Inoculation of the bacteria was carried out in a biohazard cabinet andthe procedure was based on the method described by Ram Kumar andPranay [14,16]. Approximately 1 ml of the ready bacterial broth weretransferred into mini centrifuge tubes. A sterile cotton swap wasdipped into the mini centrifuge tube containing bacteria broth andstreaked over entire of the agar plate surface, performed in 4 differentdirections. The agar plate was then left for 5-10 min before applyingthe test samples. The disc used was 6 mm diameter. A volume of thetest samples of concentration 25 ppm, 50 ppm, 100 ppm, 250 ppm,500 ppm and 1000 ppm were each pupated onto the discs and placedonto the agar plate by using sterile forceps and gently pressed toensure contact. Next to be placed on the agar plate was the discpupated with methanol as a negative control, followed by 30μg oftetracycline as standard antibacterial agent (positive control). Theplates were left at room temperature for 10 min to allow the diffusionof the test samples and the standards into the agar. Each crude extractwas tested in triplicate for each bacterium used. The plate sampleswere then incubated at 37°C for 24hrs before the inhibition zonearound every sample disc being examined. The inhibition zone wasmeasured in diameter to indicate the presence of antibacterial activityfor each sample, as compared to the positive control(Table 1).
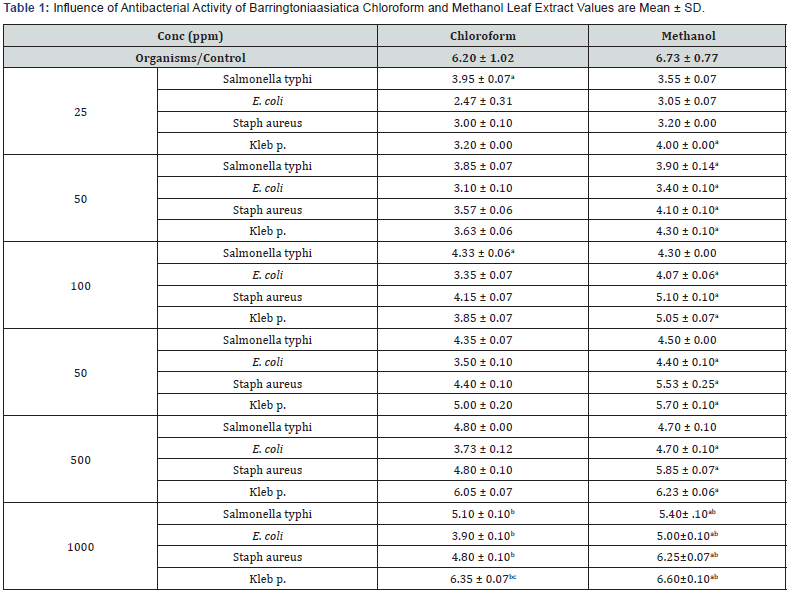
aSignificantly (p< 0.05) higher compared to different extract on same organism in each row
bSignificantly (p< 0.05) higher compared to at the same organism at different concentration in each column
cSignificantly (p< 0.05) higher compared to control in each column
Results
The antibacterial activity of chloroform and methanol extract of Barringtonia asiatica, against pathogenic bacteria both Gram-positive and Gram-negative bacteria are presented in the table above. The result of the two extract revealed that all the four pathogenic bacteria Escherichia coli, salmonella typhi, staphylococcus aureus and Klebsielia pneumonia, show much significant activity with the exception Escherichia coli, and salmonella typhi, which showed less activity when compared with the two at the same concentration. This indicated that both extract concentration of chloroform and methanol inhibited the growth of all the tested pathogens, however maximum inhibition was observed in staphylococcus aureus at concentration 500ppm and 1000ppm followed by Klebsielia pneumonia respectively.It also observed that still it exerts some degree ofsensitivity on Gram-negative bacteria. Both the concentration extracts werehighly sensitive for Gram-positive bacteria and Gram-negative bacteria Even thoughthey showed maximum inhibition for negative and positive bacteria.The result indicated that staphylococcus aureus and Klebsielia pneumonia is more sensitive in all the solvent concentration compared to the zone of inhibition of the Escherichia coli and Salmonella typhi. the data revealed that significant inhibitory activity was observed in all the pathogen.
Discussion
Plants are important source of potentially useful structures for the development of new drug agents for diseases and ailment. This can be possible through test such as in vitro antimicrobial activity assay [17].Thus, to identifying the active principle responsible for such activities and in the developing drugs for the therapeutic use in human beings. Recently much attention has been directed toward plant extracts and biologically active compounds isolated from different plant species. The use of medicinal plants plays a vital role in covering the basic health needs in developing countries and these plants may offer a new source of antibacterial, antifungal and antiviral agents with significant activity against infective microorganisms [18,19].
In the present study, the chloroform and methanol leaf extract of Barringtonia asiatica have been effectively proven for their utilization as source for antimicrobial compounds. Thus, had significant in vitro antimicrobial activity.This implied that the gram-positive and Gram-Negative bacteria were all susceptible to the extract, possibly the extract was able overcome the presence of outermembrane that serves as an effective barrier in gram negative species [20-22]. As well as the gram-positive barrier. In addition, since the zone of inhibition especially in 1000ppm for chloroform ad methanol extract (6.35±0.07mm) klebsielia pneumonia and Staphylococcus aureus (6.25±0.07mm) is almost equal to the standard (6.20±1.02mm and 6.73±0.77mm) respectively, it shows that the test organisms are sensitive to the plant extract. Thus, it may be attributed to the effectiveness of the bioactive compounds activity breaking bearer on the membrane of the organism which makes it more accessible to permeation.
The present study suggests that the solvent extraction was suitable to verify thevariation in the functional properties of medicinal plants, that was supported by many researchers [23- 26], it also justifies the claimed uses of the plant in the traditional system of medicine to treat various infectious diseases caused by the microbes.
The obtained results may provide a support of the use Barringtonia asiatica in traditional medicine. Based on this, further chemical and pharmacological investigations to isolate and identify phytochemical constituents in Barringtonia asiatica may be recommended.
Conclusion
In the present investigationIt can be concluded that most fraction of solvent extract of medicinal plant Barringtonia asiatica showed potential antimicrobial activities against the tested bacterial strain. The antimicrobial activities may be due to strong occurrence of active compounds i.e. saponins, tannins, alkaloids, steroids, phenols and flavonoids. Results of our findings confirmed the use of Barringtonia asiatica as traditional medicine. However, these medicinal plant species may be subjected to detailed phytochemical and pharmacological studies in order to find out new drugs against pathogenic bacterial.
References
- Bakar MFA, Mohamed M, Rahmat A, Fry J (2009) Phytochemicals and antioxidant activity of different parts of bambangan (Maningerapajang) and tarap (Artocarpusodoraitissimus). Food Chem 113: 479-483.
- Brunet JMC, Cetkovic GS, Djilas SM, Tumbas VT, Savatovic TSS, et al. (2009) Radical scavenging and antimicrobial of horsetail (Equisetum arvenseL.), extracts. Int. J. Food Sci. Technol 44: 269-278.
- Galeotti F, Barile E, Curir P, Dolci M, Lanzotti V (2008) Flavonoids from carnation (Dianthuscaryophyllus) and their antifungal activity. Phytochemical. Lett 1: 44-48.
- Mattila P, Hellstrom J (2007) Phenolic acids in potatoes, vegetables, and some of their products. J. Food Composition Analysis 20: 152- 160.
- Kabir MZ, RahmanSM, Islam MR, Paul P K, Rahman S, Jahan R, &Rahmatullah M (2013)A Review on a Mangrove Species from the Sunderbans, Bangladesh: Barringtoniaracemosa(L.) Roxb. Am-Eur J Sust Agric 7: 356-372.
- Barwick M (2004) Tropical and Subtropical Trees- A worldwide encyclopaedia guide. Thances and Hudson London.
- Hans ST (1998) Medicinal plant in south pacific. ISBN 9290611189 World Health Organization.
- Ravikumar T, Dam-Roy S, Krishnan P, Sankaran M,Sachithanandam V (2015) Traditional usages of ichthyotoxic plant Barringtoniaasiatica (L.) Kurz. by the Nicobari tribes. Journal of Marine and Island Cultures, 4(2): 76-80.
- Smith RL, Cohen SM, Doull J, Feron VJ, Goodman JI (2005) A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chemistry and Toxicology 43: 345-363.
- MojicaERE, MicorJRL (2007) Bioactivity study of Barringtoniaasiatica (Linnaeus)kurz. seed aqueous extract in Artemia salina. International Journal of Botany3(3): 325-328.
- Jianshe H, Xiaoyan L, Mingjun Z,Si Z (2004) A survey of the chemical constituents and pharmacological activities of mangrove medicinal plant (Barringtonia). Natural Product Research and Development16(2):167-171.
- Tona L, K Kambu, N Ngimbi, K Cimanga,AJ Vlietinck (1998) Antiamoebic and phytochemical screening of some Congolese medicinal plants. J Ethnopharmacol 61: 57-65.
- Isaac John Umaru, Fasihuddin A Badruddina, Zaini BAssim, Hauwa A Umaru (2018) Antimicrobial properties of Leptadeniahastata (Pers) Decne leaves extract. International Journal of Pharmacy and Pharmaceutical Sciences 10(2):149-152.
- Ram Kumar P, Pranay J (2010) Comparative studies on the antimicrobial activity of black pepper (Pipernigrum) and turmeric (Curcuma longa) extracts. Int J ApplBiol Pharm Technol1:491-501.
- Mahesh B, Satish S (2008) Antimicrobial activity of some important medicinal plant against the plant and human pathogens. World J Agric Sci 4:839-843.
- Vandepitte J, Engback K, Piot P, Heuck CC (1995) Basic microbiology procedures in clinical bacteriology. Geneva: World Health Organization p.85.
- Konoshima T, Yasudo T, Kashiwada Y, Cosentino LM, Lee KH (1995) Anti-aids Agents, 21.1 Triterpenoid Saponins as Anti-HIV Princi-ples from Fruits of Gleditsia japonica and Gymnocladuschinensis, and a Structure-Activity Correlation, J Nat Prod58(9): 1372-1377.
- Agrawal PK (1992) NMR Spectroscopy in the Structural Elucidation of Oligosaccharides and Glycosides, Phytochemistry 3: 3307-3330.
- Burger I, Burger BV, Albrecht CF, Spies HSC, Sandor P (1998) Triterpenoid Saponins from Becium grandiflorum var. obovatum, Phytochemistry 49: 2087-2095
- Tanor, MT, Abdul Latief Abadi, Bambang Tri Rahardjo, JantjePelealu (2014) Isolation and identification of triterpenoid saponin from BarringtoniaasiaticaKurz seeds. Journal of Tropical Life Science 4(2):119-122.
- Munoz-Mingarro D, N Acero, F Llinares,JM Pozuelo, A Ga’n de, et al. (2003) Biological activity of extracts fromCatalpa bignonioides Walt. (Bignoniaceae). JEthonopharmacol 87: 163-167.
- Coelho de SouzaG, APS Haas, GL Von Poser, EES Schapoval,E Elisabetsky (2004) Ethnopharmacological studies of antimicrobialremedies in the south of Brazil. J. Ethnopharmacol90:135-43.
- Krishna, KT, CE Ranjini, VKSasidharan (1997)Antibacterial and antifungal activity of secondarymetabolities from some medicinal and other commonplant species. J Life Sci2: 14-19.
- Singh I, VP Singh (2000) Antifungal properties of aqueous and organic solution extracts of seedplants against Aspergillus flavus and A. niger. Phytomorphol50: 151-157.
- Natarajan E, S Senthilkumar, FT Xavier, VKalaiselvi (2003) Antibacterial activities of leafextracts of Alangiumsalviifolium. J. Trop. MedPlants 4: 9-13.
- Natarajan D, JS Britto, K Srinivasan, NNagamurugan, C Mohanasundari, et al.(2005) Anti-bacterial activity of Euphorbiafusiformis- a rare medicinal herb. J. Ethnopharmacol102: 123-126.






























