Sensitivity of Detection by Biological Atomic Force Microscopy
Ana Maria Zaske*
University of Texas Health Science Center at Houston, USA
Submission: July 04, 2018; Published: July 10, 2018
*Corresponding Author: Ana Maria Zaske, University of Texas Health Science Center at Houston, USA, Email: Ana.M.Zaske@uth.tmc.edu
How to cite this article: Ana Maria Zaske. Sensitivity of Detection by Biological Atomic Force Microscopy. J of Pharmacol & Clin Res. 2018; 6(1): 555679. DOI:10.19080/JPCR.2018.06.555679
Abstract
High resolution technologies have been essential to identify structures that cannot be visualized with traditional optical microscopes. Although, the time-consuming methods used for sample preparation have limited the benefits of electron and transmission microscopy. A brief description of Atomic Force Microscopy principles and applications would be explained next, to communicate the advantages of using AFM methodologies in clinical and biomedical research.
Keywords: Atomic Force Microscopy; AFM; Cells; Elasticity; High Resolution; Nanotechnology; Imaging
Abbreviations: AFM (Atomic Force Microscope/Microscopy), k (spring constant), HCAEC (Human Coronary Arterial Endothelial Cell)
Introduction
The Atomic Force Microscope (AFM) is an innovative tool that goes beyond structure. The operative principle is based on sensing attraction forces between a probe and the sample surface (Figure 1). These forces can be analyzed by a formula known as the Hooke’s Law or law of elasticity which states that the applied force F equals a constant k (or spring constant of the probe) times the displacement x (deflection of the cantilever).
F = kx [1]
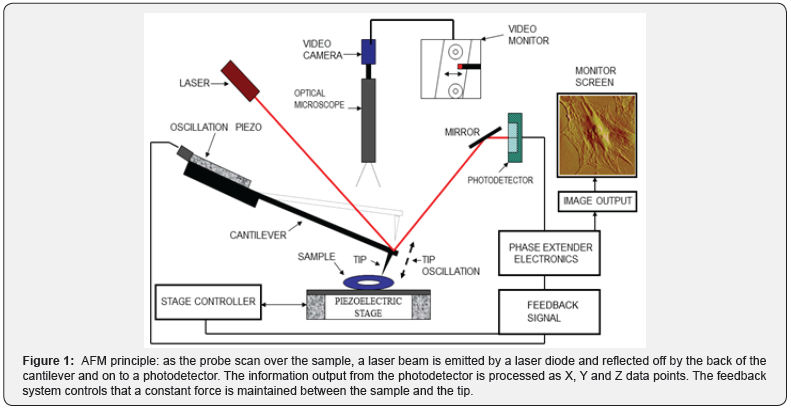
This principle governs a wide variety of applications to determine the micromechanical properties of the sample surface. Therefore, scanning is limited to analyze topography only, since internal structures are out of reach from the probe.
We have used AFM in a diversity of projects, from DNA structure to mapping elastic responses in tissue. We remark the ability of the AFM technique to analyze samples in the natural state. Living cells in media [2], fresh tissue immersed in buffer [3], or dry samples, can be tested with minimum sample preparation. It is also possible to observe the internalization process of nanovectors [4-5] and analyze their effects on the cell, or assess the development/treatment of a disease in-vitro [6]. Cell cultures are widely used to mimic biological conditions where physiological reactions take place. The effects of a treatment or disease can be assessed analyzing the changes in structure and elasticity of living cells using AFM. Some recommendations for in-vitro experiments are seeding the cell cultures to a 60% of confluency, to have individual entities in the culture and facilitate the capture of data, avoiding interference from adjacent cells. The probes have also to be flexible enough to bend when tracking the soft membrane surface. Triangular probes with a k from 0.01 to 0.1 N/m are recommended to scan living cells in media. When the structure of the cell membrane is involved, cells can be fixed with a 4% para-formaldehyde solution, to reduce drifting effects. The use of fixatives can also freeze a process on time. This is useful to analyze different stages of a treatment [4,6,7].
Differentiation, metastasis, treatment or disease can be assessed measuring the elastic response on cells and tissues, by calculating the Young’s modulus of the sample [2]. Colloidal probes have demonstrated to reproduce more reliable measurements to determine the elastic properties of soft samples. Borosilicate glass spheres, from 2-5μm in diameter, attached to silicon nitride cantilevers with k of 0.2-0.3 N/m are most recommended. When the force curves are acquired in a liquid media, the applied force to the probe should not exceed more than 15 nN in living cells. The measurements should be taken between 1 hour of the specimen been exposed to ambient conditions, to minimize variability of data. Triangular silicon nitride cantilevers with a k of 0.32 N/m are also useful to construct force volume maps in tissue sections and observe the elastic behavior of a local area [8]. Following these few suggestions, we have successfully studied physiological procedures at the nanoscale (Figure 2).

Acknowledgment
We would like to recognize the state of the art methodologies incorporated at the IM Bioscope II-AFM core facility in the University of Texas Health Science Center at Houston, for performing the AFM scanning and analyses.
References
- The Editors of Encyclopaedia Britannica (2017) Hooke’s law.
- SY Lee, AM Zaske, T Novellino, D Danila, M Ferrari, et al. (2011) Probing the mechanical properties of TNF-α stimulated endothelial cell with atomic force microscopy. International Journal of Nanomedicine 6: 179-195.
- Sahai, M Wilkerson, AM Zaske, S Olson, C Cox JR, et al. (2016) A Cost Effective Method to Immobilize Hydrated Soft Tissue Samples for Atomic Force Microscopy. BioTechniques 61(4): 206-209.
- AM Zaske, D Danila, M Queen, E Golunski, J Conyers (2013) Biological Atomic Force Microscopy for Imaging Gold-Labeled Liposomes on Human Coronary Artery Endothelial Cells. Journal of Pharmaceutics, Volume 2013, Article ID 875906, p. 8.
- R Serda, A Mack, M Pulikkathara, AM Zaske, C Chiappini, et al. (2010) Cellular association and assembly of a multi-stage delivery system. Small 6(12): 1329-1340.
- M Pulikkathara, C Mark, N Kumar, AM Zaske, RE Serda (2017) Sucrose modulation of radiofrequency-induced heating rates and cell death Converg. Sci. Phys. Oncol 3(2017): 035001.
- R Haywood Watson, JB Holcomb, EA Gonzalez, Z Peng, S Pati, et al. (2011) Modulation of Syndecan-1 Shedding after Hemorrhagic Shock and Resuscitation 6(8): 1-10.
- CA Grant, PC Twigg, DJ Tobin (2012) Static and dynamic nanomechanical properties of human skin tissue using atomic force microscopy: Effect of scarring in the upper dermis. Acta Biomaterialia 8(11): 4123-4129.






























