A New Promising Method of Hepatitis Treatment at the Level of Ultrastructure of the Liver by Standardized Powder form of Magnetite Nanoparticles (Micromage-B)
Andrey N Belousov*
Laboratory Applied Nanotechnology of Belousov, Department of Anesthesiology, Intensive care, Transfusiology and Hematology, Kharkov Medical Academy of Postgraduate Education, Ukraine
Submission: June 15, 2018; Published: July 03, 2018
*Corresponding Author: Andrey N Belousov, Department of Anesthesiology, Kharkov Medical Academy of Postgraduate Education Intensive care, Ukraine, Tel: 38050-915-18-89; E-mail: an.belousov2012@yandex.ua
How to cite this article: Andrey N Belousov. A New Promising Method of Hepatitis Treatment at the Level of Ultrastructure of the Liver by Standardized Powder form of Magnetite Nanoparticles (Micromage-b). J of Pharmacol & Clin Res. 2018; 6(1): 555676. DOI:10.19080/JPCR.2018.05.555676
Abstract
In order to determine ultra structural reconstructions in hepatic cells under effect of magnetite nanoparticles, an experiment was conducted on 37 rabbits. Micromage-B at a dose of 25 mg was dissolved in 10 ml of drinking water. Micromage-B is standardized powder form of magnetite nanoparticles. Every day the rabbit had a drink of 0.25% colloidal solution of magnetite nanoparticles for 7 days. The study ultra structural changes of hepatocytes were performed on the 8th day. Analysis of the state of submicroscopic architectonics of hepatic cells in rabbits after using Micromage-B reveals a significant activation of metabolic intracellular processes in these organs. Ultra structural organization of the liver testifies about intensification of synthetic intracellular processes, it being structurally manifested by enlargement of cisterns in the rough endoplasmic reticulum, an increased number of ribosomes, a moderate hypertrophy of the laminar cytoplasmic Goldi’s complex. For the first time in the study was the evidence of the possibility of using powder form of magnetite nanoparticles Micromage-B as an effective means of non-specific activation of metabolic processes in the liver at the ultrastructural level.
Keywords: Ultrastructure; Liver; Nanoparticles; Micromage-B
Introduction
Regenerative medicine is a pioneering field aimed at restoring and regenerating the function of damaged cells, organs and tissues in order to establish normal function. Toxicity issues are a major concern and are important factors in the context of regenerative medicine. Nanotechnology has been instrumental in the development and translation of basic research to the clinically relevant therapies. Safety is an issue of constant concern and emphasizes on the importance of investigating the issue of toxicity. Any indication of toxicity can ultimately limit the therapeutic efficiency of the therapy. Toxicity is highly dependent on the physical, chemical and structural properties of the magnetite nanoparticles (MNPs) itself as well as dose and intended use. Few in vitro studies have reported adverse effects of MNPs on cells at in vitro in therapeutic doses. However, in vivo studies have not been studied [1].
Recently, Fe3O4 magnetite nanoparticles (MNPs) have been widely used for biological applications, such as magnetic resonance imaging therapy [2], in hyperthermia tumor therapy [3], in drug delivery systems [4] and molecular detection of biomarkers in biological cells [5]. It is crucial to widely study the biological toxicity of MNPs to ensure their safety for biological and medical applications. In a literature, after 3 weeks of intravenous administration of MNPs (193nm), serum iron levels gradually increased for up to 1 week but levels slowly declined thereafter. Also, MNPs were localized in the liver and spleen parenchyma more than other tissues and cleared gradually after 3 weeks of treatment [6]. In another study, the biodistribution of intravenously injected MNPs (5nm) showed 75% of injected dose was found in the spleen and liver at 15 min post-injection. Moreover, 24% of the MNPs remain in liver after 48 hrs post-injection [2]. Consequently, as reported by Briley-Saebo (2004) MNPs were distributed equally in both liver endothelial and Kuepfer cells but not liver parenchyma following intravenous injection in rats.
After 2 days of intraperitoneal injection of MNPs (10nm), the liver showed nuclei atrophy and vacuolar degeneration in hepatocytes. Contradictory, after intravenous injection, MNPs (193nm) did not show any histological abnormalities in the liver after 7 days of treatment [6,7]. The dose and size of injected MNPs can affect the cellular and tissue toxicity in vivo and in vitro. After 2 weeks of intravenous injection of higher dose of titanium dioxide NPs (40nm), the liver showed hepatocyte degeneration, multifocal lesions, spotty necrosis of liver hepatocytes and portal lymphocyte infiltrations [8,9].
In spite of the fact that application of magnetite of nanoparticles looks simple, it is not necessary to forget about a high danger of the origins of complications as a result of their intravessel injection. At least it is necessary to take into account such indexes as a concentration, doze, rate of entered solution of nanoparticles, time of allocation nanoparticles in blood circulation after injection. The enumerated parameters for reliable have influence on haemorreology and state of microcirculation on the whole. The high local concentration of magnetite in vessels is caused by disturbances of blood circulation, microcirculation and hypoxia of tissues [10,11]. It is dangerous in main vital organs: brain, heart, lungs, liver and kidneys. Direct crosscorrelation dependence between concentration of nanoparticles and level hypoxia is physiopathology obvious. Consequently, before injecting intravessel magnetite of nanoparticles, it is necessary to have standardized water solution magnetite of nanoparticles with early studied and well-proven noninvasive physical and chemical properties.
Unfortunately, to date the advanced studies that would take into account it are absent. In the published advanced studies, we met not a single reference to the use of the early studied standardized noninvasive forms magnetite of nanoparticles and methodologies of their application. Information about the mechanism of influence magnetite of nanoparticles on main biological systems of living organism including respiratory, cardiovascular, secretory, immune systems, cellular exchange is absent. Also, we did not discover among the scientific publications of reliable dates about quantitative distribution magnetite of nanoparticles in organs and tissues after intravenous injection. Information about a mechanism of eliminate magnetite of nanoparticles from an organism is absent. On the whole, aforesaid does not allow properly estimating the scientific and practicing significance early advanced studies which were published on theme to use magnetite of nanoparticles in medicine. It was found in the choice of theme of the present investigation. The task was set in an experiment on animals to check possibility of the use of the before worked-out and studied methodology of using standardized form of magnetite nanoparticles [11-20]. The main purpose is to investigate the ultrastructural reconstructions in hepatic cells after using standardized powder form of magnetite nanoparticles Micromage-B [21-23].
Material and Methods of Research
In order to determine ultrastructural reconstructions in hepatic cells under effect of magnetite nanoparticles, an experiment was conducted on 37 rabbits. Standardized powder form of magnetite nanoparticles Micromage-B at a dose of 25mg was dissolved in 10ml of drinking water. Every day the rabbit had a drink of 0.25% colloidal solution of magnetite nanoparticles for 7 days. The study ultrastructural changes of hepatocytes were performed on the 8th day.
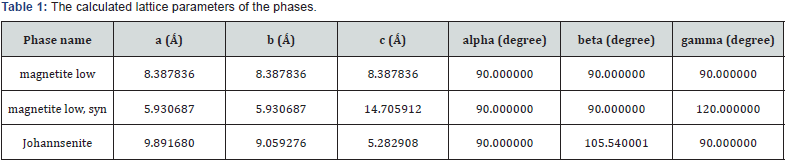
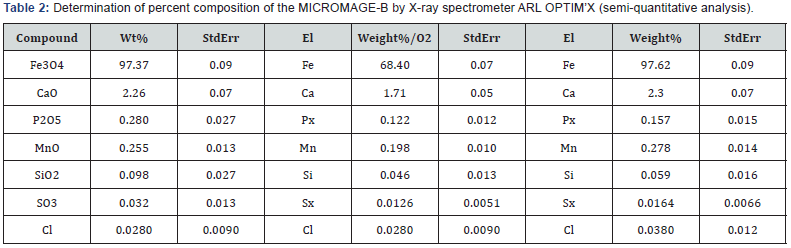
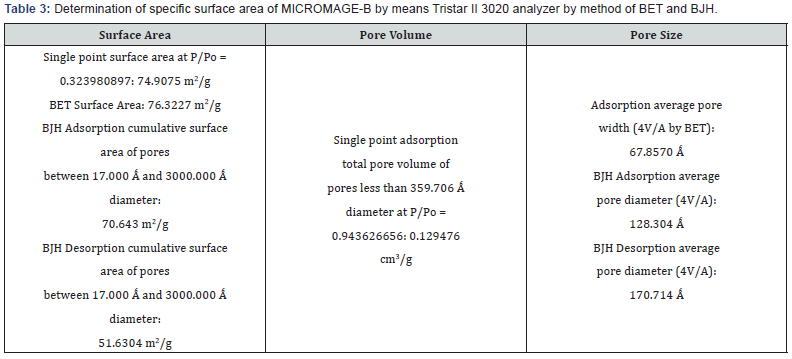
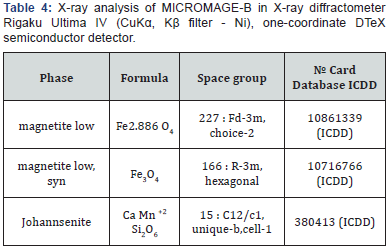
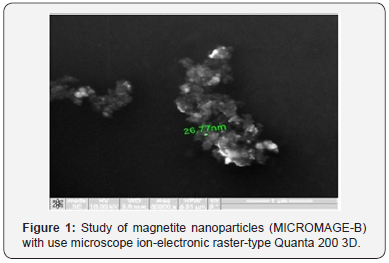
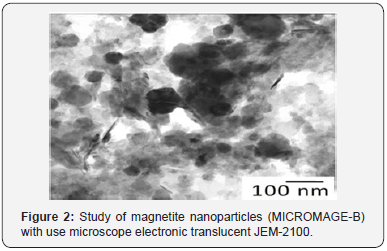
Physical and chemical properties of Micromage-B (Tables 1-5) and (Figures 1 & 2): Size of magnetite of nanoparticles is 6-12 nm;
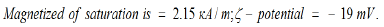
Pieces of hepatic tissue were put for preliminary fixation into a buffered (3-4%) solution of glutar aldehyde cooled down to +4 oC for 2-4 hours. After the preliminary fixation, the tissue was washed in several mixtures of the buffer at the same temperature and for final fixation it was put into 1% buffered solution of osmium tetraoxide for 2-3 hours at the temperature of +4 oC. After the end of fixation the pieces of the tissue were washed in the buffer solution, dehydrated in alcohols with rising concentrations and acetone and then embedded into a mixture of epoxy resins (epon-araldite) following conventional techniques. After polymerization in a thermostat at +60 oC, the obtained blocks were used for making ultrathin sections by UMTP-6 ultramicrotom. These ultrathin sections were contrasted by lead citrate and uranyl acetate. The preparations were studied under EMB 0-100 BR electron microscope with accelerating voltage of 75 kilovolts. Magnification was selected to be adequate for purposes of the investigation and ranged within 15.000- 45.000 times. Necessary areas of sections were photographed on photographic plates which served for subsequent taking of microphotographs. Electron microscopic preparations made out of organs of intact rabbits served as controls.
Result of researches
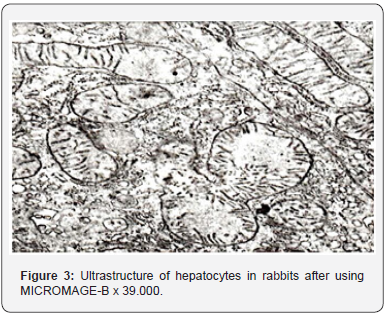
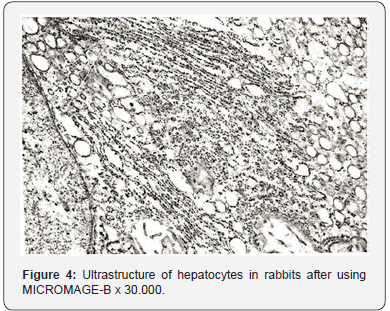
Ultra structural changes in hepatocyte organelles manifested pronounced signs of activation of reparative intracellular processes. Hepatocyte nuclei held their rounded shape (Figure 3). Nuclear membranes had well-defined contours. Chromatin, in the form of small clods, was evenly distributed throughout the section. Condensation of chromatin on the nuclear membrane was observed only in single hepatocytes. Perinuclear space was not enlarged. Single ribosome’s were found on the outer membrane of the nucleus. Mitochondria were evenly distributed in all parts of the cytoplasm of the hepatic cells (Figure 4). Mitochondrial matrix had a moderate electron density and a fine grain structure. Shape of the mitochondria varied from rounded to stick-like. Many cristae were revealed; they had a pronounced typical orientation. The outer membrane remained integral, without any foci of destruction. In single cells, there were mitochondria having the shape of dumb-bells and with septa. The rough endoplasmic reticulum underwent the most characteristic reconstructions (Figure 5).
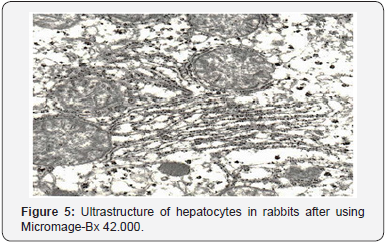
In the majority of hepatocytes, their rough endoplasmic reticulum was an extensive network of membranes with numerous ribosomes localized on their surfaces. Cisterns of the endoplasmic reticulum were slightly enlarged and their shape resembled flattened vesicles. The substance which filled them was electron transparent. The smooth endoplasmic reticulum was well developed, its vacuoles were mostly localized in basal parts of the cytoplasm. It should be noted that there were great numbers of free ribosomes and granules of glycogen which were evenly distributed throughout the cytoplasm. The laminated cytoplasmic Golgi’s complex (Figure 6) was moderately hypertrophic, its membrane part consisted of parallel smooth membranes.
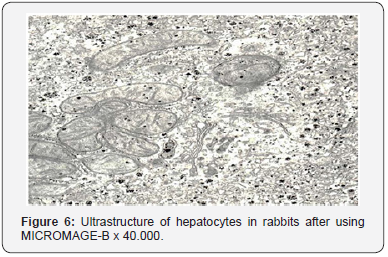
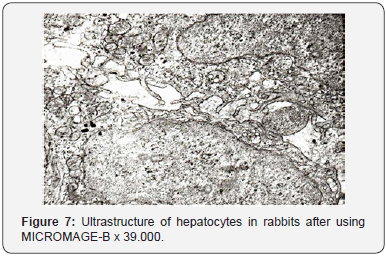
Packs of these membranes were surrounded with a great number of large and small vesicles. Single vesicles were filled with a rough fibrous osmiophil substance. There was rather a great number of primary lysosomes in the area of localization of the laminar cytoplasmic Golgi’s complex, autophagosomes and small inclusions of lipids being observed in single cells. Bile capillaries were filled with prolonged crimped microvilli and were moderately dilated. Sinus capillaries and Disse’s spaces were dilated rather extensively. Disse’s spaces were filled with numerous microvilli. Changes in the ultrastructure of Kuepfer cells testified about their functional activity. Nuclei (Figure 7) of Kuepfer cells were of irregular shape with deep invaginations of the nuclear membrane. Nuclear matrix had a significant electron density. Karyolemma had no destructive changes. The cytoplasm of Kuepfer cells revealed single and slightly swollen mitochondria which contained a small number of cristae and single cisterns of the rough endoplasmic reticulum. The cytoplasmic membrane did not undergo any changes and held its well-defined bilaminated structure. It should be noted that there was a great number of small electron-transparent micropinocytic vesicles.
Conclusion
Analysis of the state of submicroscopic architectonics of hepatic cells in rabbits after using standardized form of magnetite nanoparticles Micromage-B reveals a significant activation of metabolic intracellular processes in liver. Ultrastructural organization of the liver testifies about intensification of synthetic intracellular processes, it being structurally manifested by enlargement of cisterns in the rough endoplasmic reticulum, an increased number of ribosomes and a moderate hypertrophy of the laminar cytoplasmic Golgi’s complex. Activation of reparative intracellular processes is another aspect of these reconstructions. It is confirmed by a revealed hyperplasia of the rough endoplasmic reticulum, this hyperplasia testifying about intensive processes of self-renewal in submicroscopic structures. Presence of mitochondria having the shape of dumb-bells and with constrictions in the cytoplasm of hepatic cells enables a statement that a process of an intensive increase in the number of these organelles tares place.
Availability of a great number of mitochondria with unchanged structure and numerous cristae in the cytoplasm of hepatocytes indicated a high activity of redox processes and those of oxidative phosphorylation which satisfy needs of synthetic intracellular reactions taking place on the level of membranes and macromolecules. Submicroscopic structure of endotheliocytes in sinus capillaries indicates activation of processes of transcellular transportation of substances and electrolytes by endocytosis, it being confirmed by presence of numerous micropinocytic vesicles in the cytoplasm of these cells. Thus, for the first time in the study was the evidence of the possibility of using powder forms of magnetite nanoparticles Micromage-B as an effective means of non-specific activation of metabolic processes in the liver at the ultrastructural level. This fact should be considered from the position of promising and effectively method of hepatitis treatment by standardized nanoparticles of magnetite in clinical.
References
- Kim JS, Yoon TJ, Yu KN, Kim BG, Park SJ, et al. (2005) Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol Sci 89: 338-347.
- Markides H, Rotherham M, El Haj AJ (2012) Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. Journal of Nanomaterials 2: 11.
- Hayashi K, Nakamura M, Sakamoto W, Ozaki S, Abe M, et al. (2013) Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment pp. 366-376.
- Ansari C, Tikhomirov GA, Hong SH, Falconer RA, Loadman PM, et al. (2014) Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. 10: 566-575.
- Haun JB, Yoon TJ, Lee H, Weissleder R, (2011) Molecular detection of biomarkers and cells using magnetic nanoparticles and diagnostic magnetic resonance. Methods Mol Biol 726: 33-49.
- Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V, et al. (2008) Biodistribution clearance and biocompatibility of iron oxide magnetic nanoparticles in rats 5: 316-327.
- Alarifi S, Ali D, Al Doaiss AA, Ali BA, Ahmed M, et al. (2013) Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int J Nanomed 8: 3937-3943.
- Shanehsazzadeh S, Oghabian M, Daha F, Massoud Amanlou, Barry J Allen, et al. (2013) Biodistribution of ultra small superparamagnetic iron oxide nanoparticles in BALB mice. J Radioanal Nucl Chem 295: 1517-1523.
- AN Belousov, Patent of Ukraine Method of production of a magnetic liquid for transport and retention of medicines in organism.
- AN Belousov, Patent of Ukraine Method of treatment digestion system diseases.
- Belousov AN (2012) Effect magnetite nanoparticles MCS-B on functional activity of erythrocytes. Electrical Power & Energy Systems, China, p. 24.
- Belousov AN, Nevzorov VP (1998) Ulrastructure of hepatic cells in rabbits after injection of magnetite. / Eighth International Conference on magnetic fluids. Timisoara, Romania pp. 482-483.
- Belousov AN, Rykov VG (1998) Revealing of mechanisms of the influence exerted by magnetic fluid preparations on biological systems and the whole living organism. Plyos, p. 90.
- Belousov AN, (2000) Experimental study of effects of Belousov’s magnet-controlled sorbent on parameters of acid-base equilibrium in blood and processes of glycolysis in erythrocytes. Adsorption technologies and blood purification procedures. Germany p. 45.
- Belousov AN (2008) Opening the mechanisms of cell regulation by nanotechnology preparations. International Conference on the Scientific and Clinical Applications of Magnetic Carriers. Vancouver Canada, pp. 234-235.
- Belousov AN, (2011) The influence of magnetite nanoparticles (MCS-B) on the hemolysis of erythrocytes World Conference and Expo. TechConnect World is the host of the Nanotech BioNanotech Microtech Clean Technology and TechConnect conferences. June 13-16, in Boston, Massachusetts, U.S.A Manuscript number pp. 155.
- Belousov AN (2000) Application of products nanotechnology at creation of devices “An Artificial Liver”. 12-th World Congress of Anesthesiologists Monreal Canada, pp. 307.
- Patent of Ukraine, Method of treatment diseases which connected with blood circulation disturbance.
- www.nanolab.com.ua
- Therapeutic and preventive product MICROMAGE-B. Ukraine.
- Xu J, Shi H, Ruth M, Lazar L, Zou B, et al. (2013) Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS One 8: e70618.
- Yallapu MM, Othman SF, Curtis ET, Brij K Gupta, Meena Jaggi, et al. (2011) Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 32: 1890-1905.






























