Interaction of Herbal Formulation with Conventional Drug in Expression to Platelets Aggregation
Ankita Singh*1, Pushpendra Kannojia1, Priya Mishra1, Yogendra Singh2, Mohd Nazish Ansari2 and Mohd Asif Khan2
1Research scholar, Department of Pharmacology, IPS College of Pharmacy, India
2Bareilly International University, College of Pharmacy, India
Submission: March 05, 2018; Published: April 30, 2018
*Corresponding author: Mohd Asif Khan, Bareilly International University, College of Pharmacy, India, Email: asiikhanpcol@gmail.com
How to cite this article: Ankita Singh, Pushpendra Kannojia, Priya Mishra, Yogendra Singh, et al. Interaction of Herbal Formulation with Conventional Drug in Expression to Platelets Aggregation. J of Pharmacol & Clin Res. 2018; 5(3): 555665. DOI: 10.19080/JPCR.2018.05.555665
Abstract
Aim: To evaluate interaction among the herbal formulation with conventional drug in the treatment of platelets aggregation in rodents.
Material and Methods: Garlic powder is an herbal formulation used in ayurvedic system of medicine for the treatment of platelets aggregation. Present study deals with the interaction study of garlic with heparin. Interaction was studied in influence to platelets aggregation effect of standard drug in various parameters by inducing aggregation in male Wister rats (250-300g) and mice (25-35g). The study also involves the standardization of selected herbal formulation with various parameters like ash value, extractive value, moisture content, PH, and phytochemical screening. Acute toxicity studies (250mg/kg, 500mg/kg, 750mg/kg, 1000mg/kg, and 2000 mg/kg) orally of test sample were conducted to safe dose as per OECD- 423 guidelines. Five groups of animals (n=6) were used in this study. Group I was disease control group , Group II received Garlic powder (test drug 250mg/kg) , Group III received Garlic powder (test drug 500mg/kg), Group IV received Heparin (20μg/ml) and Garlic powder and Group V was kept s standard drug (Heparin ) treated.
Result: No sign of toxicity was observed during toxicity study of Forty days. Animals were found with normal diet feeding and normal behavioral responses. Data of this method was analyzed by ANOVA and Dunnett's multiple comparison at p<0.01.
Conclusion: The result suggests that there exist an interaction between conventional heparin and herbal formulation which was negative type.
Keywords: Platelets Aggregation; True Garlic Powder; Heparin; Hematological Parameters
Abbreviations: PRP: platelet rich plasma; PPP : platelet poor plasma; ADP: adenosine-5'-diphosphate .
Introduction
Whenever two or more drugs are being taken, there is a chance that there will be an interaction among these drugs. These interactions may increase or decrease the effectiveness or may cause serious fatal reactions. The likelihood of drug interactions increases as the number of drugs being taken increases. Therefore, people who take several drugs are at the greatest risk for interactions. Most of the possible interactions may be classified in two major categories: Pharmacokinetic and Pharmacodynamic interactions. Pharmacokinetic interactions are those that can affect the processes by which the drug increases or decreases ADME interactions4 i.e. The Absorption of a drug into the body, Distribution of the drug within the body, Alterations made to the drug by the body (Metabolism), Elimination of the drug from the body [1].
Garlic (Allium sativum) is one of the most commonly used spices throughout the world. . As a blood-thinning agent, it elevates bleeding tendency when used with NSAIDs, anticoagulants like aspirin, warfarin. Platelet aggregation plays a critical role in the pathogenesis of acute coronary syndromes, and there is extensive evidence that antiplatelet therapy reduces cardiovascular disease risk. TS Sandozi Tasneem reported interaction of herbal drugs with allopathic medicines have been reported. Since a long time herbs are being used as a medicine to cure diseases. Many persons used herbal medicines with allopathic medicines simultaneously without informing to the docter, this can lead to herb drug interaction in the body. Neeraj w have revealed the phytochemical screening of aqueous extract of various developing stages of garlic such as root, pod, and shoots was conducted then presence of flavonoids, monosaccharides, glycosides, saponins, tannins, terpenoids, and free reducing sugars was evaluated. P.V. kadamet have reported that allium sativum belongs to the family of liliaceae which contain various sulphur containing compounds such as allin, allicin, ajoene, diallylsulphide, diallyldisulphide, diallyltrisulphide, vinyldithines, ajoene, is known as inhibitor of platelet aggregation.
The study was planned with an objective to evaluate the interaction in efficiency of the herbal formulation with allopathic medicine. For the treatment of disease initially the study evaluates the efficiency of available formulation in rat as well as acute toxicity if any. The research work restricted to cardiac and blood related disorders and is supported by animal model.
The whole study was planned into two parts as shown below:
a) Evaluation of acute toxicity of interaction of garlic and heparin.
b) Pharmacological evaluation of interaction of garlic and heparin for its antiplatelet aggregation activity.
Material and methods
Chemicals and Drugs
Garlic root powder, aspirin, adenosine di-phosphate, trisodium citrate, thrombin, a-naphthol, sulphuric acid, Biuret reagent , Wagner reagent , Hager's reagent, Folin-Ciocalteu's reagent, Ninhydrin , chloroform, lead acetate, gelatin solution, magnesium ribbon, hydrochloric acid, flavonoids., chloride, phenolic content, methanol, ethanol. All the chemicals and solvents of pure and analytical grade were procured and used for the study. The purity of drugs was the priority of the research.
Animals
Healthy adult male Wister rats (250-300 g) and mice of either sex weighing between 25-35g were used for the study The animals were housed at standard experimental conditions of temperature (25±1°C) with relative humidity 50±55% under 12 h light: dark cycle. They were fed with standard rodent chow (Amit trading corporation, JayendraGanj, Gwalior, India) and water ad libitum. Experiments were performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA) after the approval of the experimental protocol by the Institutional Animals Ethical Committee (IAEC).
Heparin: 20μg/kg, Garlic powder: 250mg/kg [2].
Dose of garlic powder and heparin calculated for rats in the present study from the available therapeutic dose for the human use. The therapeutic dose was calculated by using dose conversion Table [3].
Extraction
4 gm powder drug formulation was taken randomly for extraction with suitable solvent system after examining the solubility property of the drugs for that ten batches were prepared. Hydro alcoholic solvent system was the preferable solvent system for the study as per the available literature. Simple maceration technique of extraction was followed to get the purified drug for further investigations [4].
Preliminary Phytochemical Study
Determination of Ash Value: The determination of ash value is meant for detecting low-grade products, exhausted drugs and sandy or earth matter. It can also be utilized as a meant of detecting the chemical constituents by making use of water-soluble ash and acid insoluble ash [5].
a) Total Ash Value: Air dried powder of formulation was weighed in a silica crucible and incinerated at a temperature not exceeding 450°C until free from carbon, cool and weighed and then the percentage of total ash with reference to the air dried powdered drug was calculated.
b) Acid Insoluble Ash: The ash obtained in the above method was boiled for 5 minutes with 25 ml of dilute HCL. The residue was collected on ash less filter paper and wash with hot water, ignited and weighed. The percentage of acid insoluble ash was calculated with reference to the air dried drug.
c) Water Soluble Ash: The ash obtained on total ash was boiled for 5 minutes with 25 ml of water. The insoluble matter was collected on ash less filter paper and washed with hot water and ignited to constant weight at a low temperature. The weight of insoluble matter was subtracted from the weight of the ash. The percentage of water soluble ash with reference to the air dried drug was calculated.
Loss on Drying (Moisture Content): Loss on drying is the loss in weight in % w/w resulting from water and volatile matter of any kind that can be driven off under specified conditions. A glass-stoppered dried shallow bottle was weighed. To the bottle specific quantity of sample was transferred, bottle and its contents were weighed. The sample was distributed as evenly as practicable by gentle sidewise shaking to a depth not exceeding 10 mm. the loaded bottle was placed in the oven and stopper was removed. The sample then dried to the constant weight for the specific time. The bottle and content was weighed.
Determination of Extractive Value
Extractive value is the amount of active constituents extracted with solvents from a given amount of medicinal plants material.
a) Alcohol (Methanol) Soluble Extractive: Four grams (04 g) of coarsely air dried powered of garlic containing drugwas macerated with 100 ml of alcohol in a closed flask for 24 hr., shaking frequently for 6 hr. and allowed to stand for 18 hr. It was then filtered rapidly taking precaution against loss of alcohol. 25 ml of the filtrate was evaporated to dryness in tarred flat bottomed shallow dish, dried at 105°C and weighed. The percentage of alcohol soluble extractive was calculated with reference to the air dried drug.
b)Water (Distilled) Soluble Extractive
4 g of coarsely air dried powered of drug containing garlic was macerated with 100 ml of chloroform water in a closed flask for 24 h, shaking frequently for 6 h and allowed to stand for 18 hr. it was then filtered rapidly taking precaution against loss of chloroform water. 25 ml of the filtrate was evaporated to dryness in tarred flat bottomed shallow dish, dried at 105°C and weighed. The percentage of alcohol soluble extractive was calculated with reference to the air dried drug.
Determination of PH Value: The pH value of an aqueous liquid may be defined as the common logarithm of reciprocal of the hydrogen ion concentration expressed in gram per liter. The pH value of liquid was determined potentially by means of the glass electrode and a suitable pH meter.
Phytochemical Screening
Preliminary phytochemical screening of the formulation was carried out to identify the presence and absence of various phytoconstituents like flavonoids, phenolic compounds, alkaloids etc present in the formulation [5].
Test for Carbohydrates
Molish Test: To 2-3 ml test sample, few drops of a-naphthol solution was added and shaken. Then conc. sulphuric acid was added from sides of test tube to observe the formation of violet ring at the junction of two liquids.
Tests for Protein
a) Biuret Test: To 3ml of sample, Biuret reagent was added. The formation of violet color was observed for the presence of protein.
b) Xanthoproteic Test: To 2ml of extract 1ml of conc. H2So4 were added. The formation of white precipitate was observed for the presence of protein.
Tests for Amino Acids
Ninhydrin Test (General Test): 3ml of extract and 3 drops 5% Ninhydrin solution was heated in boiling water bath for 10 min. Appearance of purple or bluish color was observed.
Tests for Alkaloids
a) Wagner's Test: To2-3 ml of filtrate, few drops of Wagner's reagent was added. The formation of reddish brown precipitate was observed for the presence of alkaloids.
b) Hager's Test: To 1 ml of sample and few drops of Hager's reagent (saturated picric acid solution) were added. The formation of yellow colored precipitate was observed for the presence of alkaloids.
Tests for Glycosides
Kellar Kiliani Test: To 2 ml of sample, 2 ml of glacial acetic acid with one drop of ferric chloride and concentrated sulphuric acid was added. Formation of reddish brown color at junction of two liquid layers with upper bluish green was observed for the presence for glycosides.
Test For Saponins
Foam Test: The drug extract or dry powder was vigorously shaken with water observed for formation of persistent foam.
Tests for Sterols
Salkowski test: To 2ml of extract, 2ml chloroform and 2ml concentrated H2SO4 were added and shaken well. Formation of red colour in chloroform layer and greenish yellow fluorescence in acid layer was observed.
Tests for Tannins and Phenolic Compounds
a) Ferric Chloride Test: To 2-3 ml of sample and few drops of 5% chloroform solution should added. Formation of bluish black color was observed for the presence of phenols.
b) Lead Acetate Test: To 2-3 ml of sample and added few drops of lead acetate solution was added. Formation of white ppt. was observed.
c) Gelatin Test: To 2-3 ml of sample and add few drops of gelatin solution was added. Formation of white ppt. was observed.
Tests for Flavonoids
Shinoda Test (Magnesium Hydrochloride Reduction Test): To 2ml of the test sample, few fragments of magnesium ribbon and conc. hydrochloric acid was added drop wise and heated gently. The formation of reddish to pink color was observed for the presence of flavonoids.
Characterization Of Drug
Thin Layer Chromatography: Separation of compounds bioactive compounds from garlic extract was separated by using TLC technique. Solvent system consisting of butanol: acetic acid: water (12:3:5) respectively was used. Garlic extract was diluted with sterilized distilled water (10%) and spotted on the TLC plates using capillary tube. Plates will be run in a chamber containing the solvent system. Plates were dried at room temperature. Plates was then developed using ninhydrin. RF value for each band was calculated.
Pharmacological Investigation
Induction of Platelet Aggregation in Rats: Platelet aggregation in rat PRP will be monitored according to the protocol described earlier. To assess effect of highly lipophilic agents, compound was administered via intra peritoneal or oral route 60 min prior to blood collection. Rats were anaesthetized with ether and blood (9ml) was drawn from the heart into a plastic syringe containing 1 ml of 1.9% tri- Sodium citrate. The blood was centrifuged at 300g for 20 min and the platelet rich plasma (PRP) was collected. The remaining blood was further centrifuged at 2500g for 15 min at 20°C to obtain platelet poor plasma (PPP). The platelet count in the PRP was adjusted to 2X108cells/ml by using PPP. Aggregation was induced by adenosine-5'-diphosphate (ADP), thrombin, collagen, or calcium ionophore A23187 and PMA was monitored on a dual channel aggregometer. Minimum of 4 numbers of observations were recorded for each experiment. Percent inhibition of the test groups were calculated as follows:

Where, Aggregation vehicle= Aggregation obtained in vehicle/control group and Aggregation test= Aggregation obtained in test group.
Justification for Dose Selection: Dose of true garlic powder & heparin was calculated for rats in the present study from the available therapeutic dose for the human use. The therapeutic dose will be calculated by using dose conversion table and as per the literature [3].
Toxicity Study: Animals: Healthy adult Wister albino rats (200-250g) and Swiss albino mice (28-35g); individually in polypropylene cages, maintained under standard conditions (12 h light and 12 h dark cycle; 25± 30°C; 35 - 60% humidity). The animals were fed with standard rat pellet diet and water ad libitum.
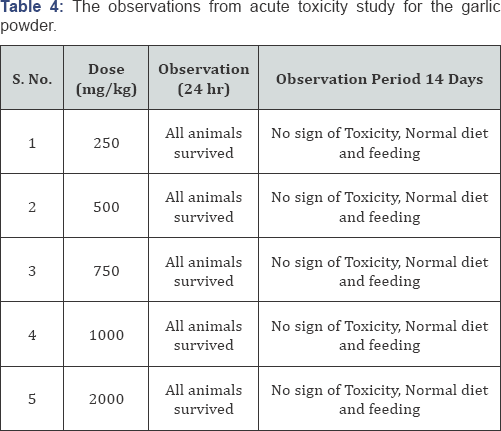
Acute Toxicity Study: Acute toxicity studies were conducted to determine the safe dose as per OECD-423 guidelines. Drugs will be administered orally to overnight fasted animals. After drug administration the animals were observed continuously for 1 hour, frequently for the next four hours, and then after 24 hours. After administration, Irwin's test was conducted, where the animals were observed for gross behavioral changes. The toxic dose was determined by observing the mortality rate in the drug treated groups. From this the therapeutic dose was selected for the further study.
In-Vivo Study: The animals were caged and divided into six groups, and each group contains five animals. The cages were labeled properly with number, age and sex of the animals. The animals were marked with permanent marker on tail for identification. The groups are as follows:
Group I : Control
Group II : Treatment with herbal formulation (Dose I)
Group III : Treatment with herbal formulation (Dose II)
Group IV : Treatment with Herbal Formulation + Standard drug
Group V : Treatment with standard drug (heparin)
Parameters
Bleeding Time: Bleeding time in mice was evaluated by the method of Dejana, et al. (1979) [6]. The tail 2mm from tip of mice was incised and the blood oozed was soaked on a filter paper, which was monitored at an interval of 10-15 sec till the bleeding stops. The time elapsed from the tip incision to the stoppage of bleeding was determined as the bleeding time. The CDRI compounds (30~M/kg), aspirin (30mg/kg) or vehicle was given orally 60 min prior to the tail incision in a group of 5 mice each.
Biochemical Parameters: On 20th blood was withdrawn through retro orbital vein puncture of all groups and the biochemical parameters were analyzed. Hemoglobin content was estimated by the method of Drabkin and Austin [7] red blood cell and white blood cell counts were estimated according to the method of Chesbrough and MC Arther [8] in an improved neubauer chamber. Estimation of erythrocyte sedimentation rate will be followed by the method of westergren [9].
Statistical Method
The statistical analysis of the data was performed using the Dunnet's test and one way analysis (ANOVA) with the help of available update software technology. The results were analyses and the test groups were compared with the standard data. Difference between groups was compared by using One-way ANOVA followed by Dunnett's Multiple Comparison. P<0.05 was considered significant.
Results And Discussion
The percentage yield of extracted herbal formulation calculated by using formula:
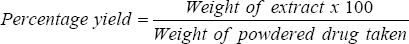
% Yield = 41.2%
Standardization of Obtained Drug (Table 1)
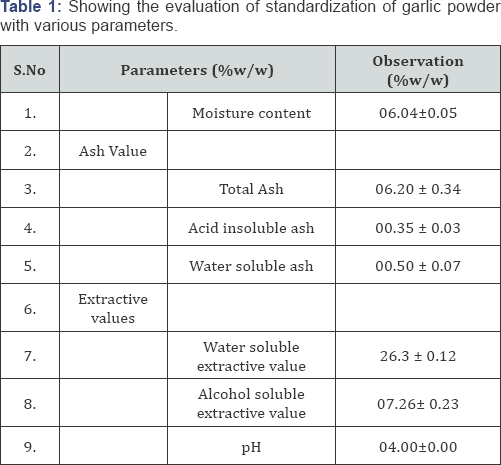
*Values are expressed as mean ± standard deviation.
Phytochemical Screening (Table 2)
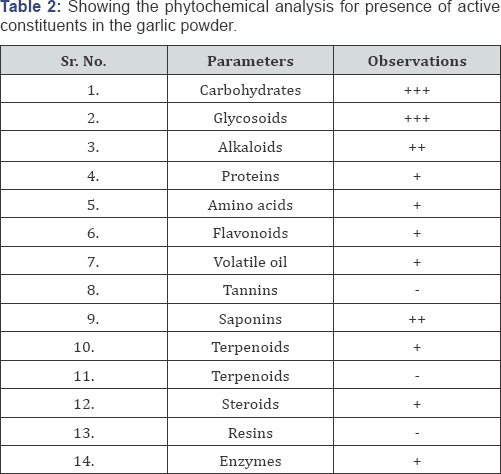
- Absent; + low concentration; ++ medium concentration; +++ high concentration.
Thin Layer Chromatography (TLC) (Table 3)

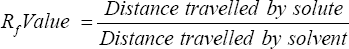
Pharmacological Study
Acute Toxicity Study (Table 4)
Pharmacological Evaluation (Table 5)
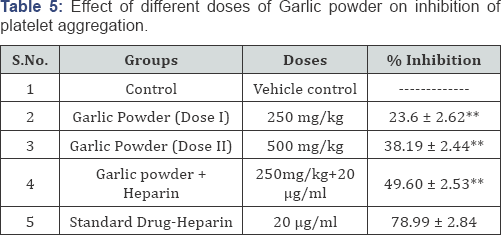
Values are expressed as mean ± SEM. P<0.01 compared to standard (n=6)
Haematological Evaluation (Table 6)
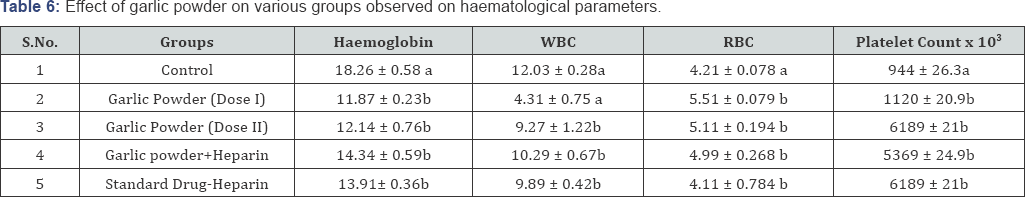
a,b = Means with different superscripts (a,b) are significantly different (P < 0.05) when compared to control group
Conclusion
Platelets play a vital role in health and diseases given their central involvement in homeostasis and thrombosis. Platelet aggregation is absolutely essential for the formation of hemostatic plug when normal blood vessels are injured. Garlic powder may have an antiplatelet function and make the blood thin. In the present investigation the administration of garlic powder with calculated dose reveals there is a percentage of inhibition in a dose dependent manner. As the dose increases percentage inhibition among the platelet aggregation increases. In combination treatment both the heparin and garlic powder there is a increase percentage inhibition of platelet aggregation when compared with individual administration of heparin and garlic powder.
In conclusion many cardiovascular diseases can be attributed to excessive platelets aggregation which has a critical role in thrombus formation. It appears that garlic powder can 2. inhibit platelets aggregation in-vitro therefore it may be used to present or in the treatment of some cardiovascular diseases 3. and blood related disorders. The present study ensures the platelet aggregation of garlic powder that may be beneficial 4. for the cardiovascular disorder patient. However the drug 5 should be used with precautions by patients with bleeding or other heamotological disorders as the combined use of garlic 6. with allopathic medicine may increase the risk of bleeding and heamotological complications.
Acknowledgement
I am very thankful to my guide Dr. Pushpendra Kannojia without whom it could not be completed. And I am also grateful to IPS- College of Pharmacy, Gwalior for its great appreciation and issuing all the things needed in my project.
References
- P manoj kumar, Siddhanand S Kulkarni, Shashikant d Wadkar (2014) Review on interaction of herbal medicines with allopathic medicines 2(2).
- Kulkarni SK (1999) Handbook of experimental pharmacology. (3rd edn.); Vallabh Prakashan, New Delhi, India.
- Paget GE, Barnes JM ( 1964) Evaluation of drug activities, Pharmacometrics, Academic press, New York, USA, pp. 161.
- Kokate CK (2005) Pharmacognosy (13th edn.); Nirali Prakashan, Pune.
- Khandelwal KR (2008) Practical Pharmacognosy (4th edn.); Pragati Books, India. pp. 220.
- Dejana E, Calliono A, De Gaetano G (1979) Bleeding time in laboratory animals: II - a comparison of different assay conditions in rats. Thromb Res 15(1): 191-197.
- J Harold Austin, David L (1935) spectromectric studies "methemoglobin”. The Journal of Biological Chemistry 112: 67-88.
- Chesbrough and MC Arther (1998) Erythrocyte sedimentation rate, postgraduate medicine. 103(5).
- Zlonis M (1993) The mystique of the erythrocyte sedimentation rate, a reappraisal of one of the oldest laboratory tests still in use 13(4): 787-800.






























