CD22ΔE12 as a Molecular Target for RNAi Therapy in Mantle Cell Lymphoma
Fatih Uckun*1-4 and Sanjive Qazi5
1AresMIT Biomedical Computational Strategies (ABCS), USA
2Ares Pharmaceuticals, LLC, St. Paul, USA
3Division of Hematology Oncology, Department of Pediatrics, University of Southern California, USA
4Norris Comprehensive Cancer Center, University of Southern California Keck School of Medicine, USA
5Bioinformatics Program, Gustavus Adolphus College, St. Peter, USA
Submission: December 20, 2017; Published: January 08, 2018
*Corresponding author: Fatih Uckun, AresMIT Biomedical Computational Strategies (ABCS), USA; Email: fatih.uckun@aresmit.com
How to cite this article: Fatih U, Sanjive Q. Cd22ΔE12 as a Molecular Target for RNAi Therapy in Mantle Cell Lymphoma. J of Pharmacol & Clin Res. 2018; 4(5): 555650.DOI:10.19080/JPCR.2018.04.555650
Editorial
Non-Hodgkin lymphoma (NHL), is the most common lympho hematopoietic malignancy in the US with more than 70,000 new cases that contribute to more than 20,000 deaths per year (American Cancer Society: Cancer Facts and Figures 2017. Atlanta, GA: American Cancer Society, 2017). Mantle cell lymphoma (MCL) represents a subgroup of poor prognostic, high- risk NHL with an aggressive biology and short progression-free as well as overall survival that usually occurs in middle-aged or older adults (Median age: 68 years) [1-3]. MCL is a clinically and molecularly heterogeneous CD 19+ B-lineage NHL comprising approximately 7 percent of adult NHL cases [1-28]. More than two thirds of the MCL patients present with advanced stage disease at diagnosis requiring treatment with a combination of chemotherapy and immunotherapy [11-15,17-18,25]. There is no evidence that any of the currently available treatment regimens can cure MCL and almost every MCL patient eventually develops therapy-resistant refractory disease. Therefore, identification and evaluation of non-cross resistant active new agents and new drug combinations against MCL, including exploratory single agent Phase II clinical studies in relapsed or refractory MCL, as well as more intense multimodality strategies with immuno- chemotherapy and hematopoietic stem cell transplantation for eligible patients, have been among focal points in translational lymphoma research [1-3,10,16-27]. The insights provided by translational and clinical research efforts have resulted in improvement of the overall survival in MCL and increased the number of available salvage treatment options for patients with recurrent disease [1,2]. New agents with promising single agent activity against relapsed MCL and hematopoietic stem cell transplantation protocols with less intensive conditioning regimens have been developed for treatment of relapsed MCL [20-23,26-28].
Our team was the first to identify CD22 exon 12 deletion (CD22 Δ12) as the likely genetic cause for the chemo resistance- associated activation of SYK/MAPK, PI3-K/m-TOR and WNT pathways in aggressive B-lineage lymphoma and leukemia cells [29].The CD22Δ12 genetic defect [29,30] involves CD22, an inhibitory co-receptor of human B-cells and B-cell precursors that acts as a negative regulator of multiple signal transduction pathways critical for proliferation and survival. Aggressive B-lineage lymphoma and leukemia cells express a dysfunctional CD22 due to deletion of exon 12 (CD22Δ12) arising from a splicing defect associated with homozygous intronic mutations [29,30]. Functional RNA interference (RNAi) experiments using CD22Δ12-specific siRNA and its nano scale formulations both in vitro and in vivo have confirmed the causal link between CD22 Δ12 and the stemness features as well as aggressiveness and chemotherapy resistance of human B-lineage leukemia/ lymphoma cells. Notably, forced expression of the mutant CD22Δ12 protein in transgenic (Tg) mice under control of the immunoglobulin enhancer E|i that is activated in early B-cell ontogeny prior to immunoglobulin gene rearrangements caused fatal B-lineage leukemia with lymphomatous features in C57/ BL/6 mice [30,31]. This Tg mouse model recapitulated the gene expression profile of CD22Δ12+ human B-lineage lymphoma and leukemia cells, indicating that CD22Δ12 alone as a driver lesion is sufficient for malignant transformation and clonal expansion of B-lineage lymphoid cells [29,30]. Leukemia cells from CD22 Δ12-Tg mice exhibit characteristic gene expression and protein expression profiles consistent with constitutive activation of multiple signaling networks, including the WNT, PI3-Kinase and MAPK/SYK pathways, mimicking the profiles of aggressive human B-lineage leukemia/lymphoma cells [31,32].
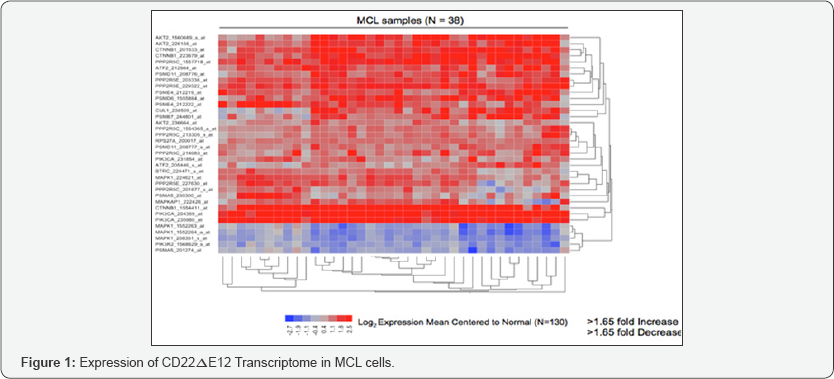
We recently reported that the CD22Δ12 defect has been detected in 97% of MCL cases [31-33]. As shown in Figure 1, the CD22Δ12 transcriptome was strongly represented in primary lymphoma cells from MCL patients. We have established a highly efficient small interfering RNA (siRNA) delivery platform based on a rationally designed cell-penetrating cationic helical polypeptide. We are now developing advanced multifunctional bioactive nanomaterials with optimized properties as siRNA delivery vehicles in an attempt to further improve the potency and broaden the therapeutic window of their nanocomplexes with therapeutic siRNA. We have reported membrane-penetrating endosomolytic hybrid nanocarriers for RNA interference therapy in poor prognosis B-lineage leukemias and lymphomas [32,33]. This nanoscale siRNA delivery platform has been further optimized to enhance its translational impact potential [32-35]. We have complexed CD22 Δ12-siRNA with a 200-mer polymer of the lead helical polypeptide to prepare a nanoscale formulation of CD22Δ12-siRNA. This unique nanoparticle formulation caused marked CD22Δ12 mRNA and protein depletion in neoplastic B-lineage leukemia and lymphoma cells and inhibited their clonogenic growth. These hybrid nano carrriers are being functionalized with a MCL targeting moiety directed against the CD19 [36] surface receptor on MCL cells in order to achieve optimal delivery to and uptake by MCL cells to further reduce their potential toxicity and improve their efficacy. Polypeptide-based siRNA nano complexes with CD 19-binding functionality could represent an important addition to the emerging new personalized treatment options for MCL.
References
- Kahl BS, Dreyling M, Gordon LI, Quintanilla Martinez L, Sotomayor EM (2017) Recent advances and future directions in mantle cell lymphoma research: report of the 2016 mantle cell lymphoma consortium workshop. Leuk Lymphoma 31: 1-12.
- Spurgeon SE, Till BG, Martin P, Goy AH, Dreyling MP, Gopal AK, et al. (2016) Recommendations for Clinical Trial Development in Mantle Cell Lymphoma. J Natl Cancer Inst 31(1): 109.
- Steven Swerdlow H, Campo E, Pileri SA, Nancy Lee Harris, Harald Stein, et al. (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127: 2375-2390.
- Jares P, Colomer D, Campo E (2012) Molecular pathogenesis of mantle cell lymphoma. J Clin Invest 122(10): 3416-3423.
- Hofmann WK, de Vos S, Tsukasaki K, Wachsman W, Pinkus GS, et al. (2001) Altered apoptosis pathways in mantle cell lymphoma detected by oligo nucleotide microarray. Blood 98: 787-794
- Nagy B, Lundan T, Larramendy ML, Aalyo Y, Zhu Y, et al. (2003) Abnormal expression of apoptosis related genes in hΔmatological malignancies: overexpression of MYC is poor prognostic sign in mantle cell lymphoma. Br J HΔmatol 120(3): 434-441.
- Rizzatti EG, Falcao RP, Panepucci RA, Proto Siqueira R, Anselmo Lima WT, et al. (2005) Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta signalling pathways. Br J HΔmatol 130(4): 516-526.
- Rinaldi A, Kwee I, Taborelli M, Largo C, Uccella S, et al. (2006) Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. Br J HΔmatol 132(3): 303-316.
- Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, et al. (2016) Prognostic Value of Ki 67 Index, Cytology, and Growth Pattern in Mantle Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 34(12): 1386-1394.
- Fenske TS, Zhang MJ, Carreras J (2014) Autologous or reduced- intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy sensitive mantle cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol 32(4): 273-281.
- Lenz G, Dreyling M, Hoster E, Wormann B, Duhrsen U, et al. (2005) Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 23(9): 1984-1992.
- Griffiths R, MikhΔl J, Gleeson M, Danese M, Dreyling M (2011) Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood 118(18): 4808-4816.
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, et al. (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 noninferiority trial. Lancet 381(9873): 1203-1210.
- Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, et al.(2014) Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 123(19): 2944-2952.
- Ruan J, Martin P, Furman RR, Lee SM, Cheung K, et al. (2011) Bortezomib plus CHOP rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol 29(6): 690-697.
- Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, et al. (2016) Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 388(10044): 565-575.
- Rummel MJ, Knauf W, Goerner M, Ulrike Soeling, Elisabeth Lange, et al. (2016) Two years rituximab maintenance vs. observation after first- line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: First results of a prospective, randomized, multicenter phase II study. J Clin Oncol p. 34.
- Ruan J, Martin P, Shah B, Stephen Schuster J, Sonali Smith M, et al. (2015) Lenalidomide plus Rituximab as Initial Treatment for MantleCell Lymphoma. N Engl J Med 373: 1835-1844.
- Hartmann EM, Campo E, Wright G, Lenz G, Salaverria I, et al. (2010) Pathway discovery in mantle cell lymphoma by integrated analysis of high-resolution gene expression and copy number profiling. Blood 116(6): 953-961.
- Samad N, Younes A (2010) Temsirolimus in the treatment of relapsed or refractory mantle cell lymphoma. Onco Targets Ther 3: 167-178.
- Dreyling M, Jurczak W, Jerkeman M, Rodrigo Santucci Silva, Chiara Rusconi, et al. (2016) Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle cell lymphoma: an international, randomised, open label, phase 3 study. Lancet 387 (10020): 770-778.
- Wang ML, Rule S, Martin P, Andre Goy, Rebecca Auer, et al. (2013) Targeting BTK with ibrutinib in relapsed or refractory mantle cell lymphoma. N Engl J Med 369: 507-516.
- Wang ML, Lee H, Chuang H, WagnerBartak N, Hagemeister F, et al. (2016) Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single centre, open label, phase 2 trial. Lancet Oncol 17(1): 48-56.
- Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, et al. (2013) Single agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 31(29): 3688-3695.
- Rummel M, Kaiser U, Balser C, Martin Stauch, Wolfram Brugger, et al. (2016) Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol 17(1): 57-66.
- Kahl BS, Spurgeon SE, Furman RR, Ian Flinn W, Steven Coutre E, et al. (2014) A phase 1 study of the PI3KS inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood 123: 3398-3405.
- Le Gouill S, Kroger N, Dhedin N, Nagler A, Bouabdallah K, et al. (2012) Reduced intensity conditioning allogeneic stem cell transplantation for relapsed refractory mantle cell lymphoma: a multicenter experience. Ann Oncol 23(10): 2695-2703.
- Morschhauser FA, Cartron G, Thieblemont C, Solal Celigny P, Haioun C, et al. (2013) Obinutuzumab (GA101) monotherapy in relapsed/ refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol 31(23): 29122919.
- Uckun FM, Goodman P, Ma H, Dibirdik I, Qazi S (2010) CD22 Exon 12 Deletion as a Novel Pathogenic Mechanism of Human B Precursor Leukemia. Proc. Natl. Acad. Sci. USA 107(39): 16852-16857.
- Ma H, Qazi S, Ozer Z, Gaynon P, Reaman GH, et al. (2011) CD22 Exon 12 deletion is a characteristic genetic defect of therapy refractory clones in pΔdiatric acute lymphoblastic leukΔmia. Br J HΔmatol 156(1): 89-98.
- Uckun FM, Qazi S, Ma H, Reaman GH, Mitchell L (2015) CD22Δ12 as a molecular target for corrective repair using RNA trans-splicing: antileukemic activity of a rationally designed RNA trans-splicing molecule. Integrative Biol (Camb) 7(2): 237-249.
- Uckun FM, Qazi S, Ma H, Yin L, Cheng J (2014) A rationally designed CD22Δ12-siRNA nanoparticle for RNAi therapy in B-lineage lymphoid malignancies. EBioMedicine 1(2-3): 141-155.
- Uckun FM, Qazi S, Ma H, Reaman GH, Mitchell L (2015) CD22Δ12 as a molecular target for corrective repair using RNA trans-splicing: anti leukemic activity of a rationally designed RNA trans-splicing molecule. Integrative Biol (Camb) 7(2): 237-249.
- Yin L, Song Z, Qu Q, Kim K, Zheng N, et al. (2013) Supramolecular selfassembled nanoparticles (SSNPs) mediate oral delivery of therapeutic TNF-a siRNA against systemic inflammation. Angewandte Chemie International Edition 52(22): 5757-5761.
- Zheng N, Song Z, Liu Y, Zhang R, Zhang R, et al. (2015) Redox responsive, reversibly-cross linked thiolated cationic helical polypeptides for efficient siRNA encapsulation and delivery. J Control Release 205: 231239.
- Uckun FM, Myers DE, Qazi S, Ozer Z, Rose R, et al. (2015) Recombinant Human CD19L-sTRAIL Protein Effectively Targets B-Precursor Acute Lymphoblastic Leukemia. J Clin Invest 125(3): 1006-1018.






























