Characterization and Clinical Applications of Amniotic Membranes
Vo AT1, Diller RB1 and Kellar RS*12
1Axolotl Biologix, Phoenix, Arizona, USA
2Northern Arizona University, Center for Bioengineering Innovation, Flagstaff, Arizona, USA
Submission: December 03, 2017; Published: December 13, 2017
*Corresponding author: Robert S. Kellar, Northern Arizona University, Center for Bioengineering Innovation, Flagstaff, Arizona, USA, Tel: 928-523-9311; Email: Robert.Kellar@nau.edu
How to cite this article: Vo AT, Diller RB, Kellar RS. Characterization and Clinical Applications of Amniotic Membranes. J of Pharmacol & Clin Res. 2017; 4(4): 555645. DOI: 10.19080/JPCR.2017.04.555645
Abstract
During pregnancy, every vertebrate has layers of extraembryonic tissue surrounding the developing fetus called placental membranes, or fetal membranes. The two fetal membranes which immediately surround the amniotic cavity are the chorion (outer membrane) and the amnion (inner membrane). The chorion is an opaque membrane which lies on the outer layer of the amniotic sac, whereas the amnion is a more translucent structure which lies on the inner layer, adjacent to the amniotic fluid [1]. These membranes have a composition of extracellular connective tissue components, making them a promising biomaterial for a variety of therapies. Additionally, the cells of these fetal membranes are a potential source of material for stem cell therapies. Transmission electron microscopy (TEM) comparisons of the ultrastructure of chorion vs. amnion stromal cells show that chorion stromal cells are similar to hematopoietic progenitor cells, while amnion stromal cells are more like mesenchymal and epithelial cells, suggesting multi-potentiality [2]. Due to the composition of extracellular connective tissue components and the stem cell properties of their epithelial and mesenchymal cells, the amnion has become highly attractive for cell-based repair and regenerative medicine, despite being traditionally considered a waste material or byproduct of pregnancy (Figure 1).
Abbreviations: TEM: Transmission Electron Microscopy; ECM: Extracellular Matrix; HA: Hyaluronic Acid; MMPs: Matrix Metalloproteinases TIMPs: Tissue Inhibitors of Metalloproteinases; ILs: InterLeukins
Structure and Function
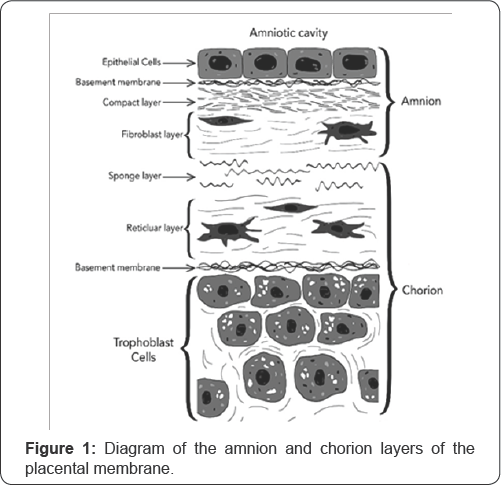
The amnion is a thin, avascular membrane, with a thickness of about 35-60 microns [2], and it is comprised of three major structures: the amniotic epithelium (i.e. epithelial layer), the basement membrane, and the avascular stroma (i.e. stromal layer, mesenchymal layer). See Figure 1 for a rendering of the amnion and chorion layers of the placental membranes and their constituent parts. The epithelial layer of the amnion is directly in contact with the amniotic fluid and nearest to the developing fetus. It is comprised of a single layer of simple cuboidal epithelial cells (i.e. human amniotic epithelial cells, or hAECs), which exhibit pluripotent stem cell-like characteristics [1].
The basement membrane is comprised of several molecules which are associated with cell differentiation and survival, such as laminins, type IV and type VII collagen and fibronectin [1]. The avascular stroma can be further subdivided into three layers: a compact layer, fibroblast layer, and spongy layer. The compact layer is located immediately outside the basement membrane and provides the primary fibrous skeleton for the amnion. The fibroblast layer consists of mesenchymal cells which have pluripotent stem cell-like qualities and are responsible for secreting type I, type III, type V, and type VI collagens which provide mechanical integrity to the amniotic membrane. The spongy layer is the outermost layer of the amniotic membrane and sits adjacent to the chorion. It is abundant in collagen, glycoproteins, and proteoglycans which cause a "spongy" appearance in histological preparations.


The two major components of the amniotic membrane are the cells and the extracellular matrix (ECM). The cells are responsible for the synthesis, degradation, and turnover of the ECM components; the ECM significantly influences cell behaviors such as adhesion, growth, and differentiation in vivo [3]. The ECM is comprised of specialized proteins like collagen, elastin, fibronectin, laminin, and proteoglycans that provide structural support to the amniotic membrane. The structure of the ECM can be seen in the scanning electron micrographs of a dehydrated human amniotic membrane at high magnification in Figures 2 & 3. In Figure 2, the extracellular matrix is a dense fibrous network primarily composed of collagen fibers. In Figure 3, the layering of the fibers is visible from a transverse view of the amniotic membrane. Despite being a simple avascular structure, the amnion is also responsible for the production and transport of a wide variety of bioactive factors such as peptides, growth factors, and cytokines. These soluble factors include hyaluronic acid (HA), matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), interleukins (ILs), migration inhibitory factor, and prostaglandins [1]. Because the ECM contains such a wide variety of structural proteins and bioactive factors, the amniotic membrane is equipped with unique morphological and biochemical properties which make it suitable for many different clinical applications.
Clinical Applications of Amniotic Membrane:
Amniotic membrane favorably influences the wound-healing process while reducing pain and discomfort for the patient [1]. The human amnion has been used in a variety of clinical applications since the early 20th century. The first reported application of amniotic membrane in medicine was in 1910, when John Staige Davis used fetal membranes (i.e. amnion and chorion) as a permanent skin transplant [4]. The use of human amnion in burn treatment dates back to 1913, and it has since been used to treat superficial and partial-thickness burns at many centers worldwide [4]. Human amniotic membrane has been used in ophthalmological treatments since the 1940s, although there were no reports on the use of the membrane in ophthalmology until 1995, which caused a resurgence of its use in the field [5]. Recently, human amnion has been widely used in ophthalmology as a biological dressing for ocular chemical burns, ulceration, necrosis, or other cases of severe inflammation [6].
Human amniotic membrane is also a promising treatment for chronic wounds, such as venous ulcers [7]. In clinical applications for wound healing, the human amniotic membrane is generally placed with the epithelial side down against a wound bed to efficiently release growth factors into the site [1]. Research is being performed to expand the use of human amnion into treatments associated with the genitourinary tract, oral cavity, stomach, cartilage repair, and brain [1]. Human amniotic membrane is an ideal candidate for a wide variety of clinical applications because of its special properties, relatively high availability, low-cost, lack of immune markers and non-invasive recovery.
Characteristics of Amniotic Membrane
The exact mechanism of action of human amniotic membrane in its many potential applications is still not fully understood, but it is probably best regarded as a physical barrier with a variety of unique biological properties which come largely from the protein composition of the ECM [5]. Because the amniotic membrane provides an extracellular matrix of structural matrix protein fibers and glycosaminoglycans [3], it promotes cellular migration and proliferation [1] by providing a 3D scaffold which acts as a physical support structure while regulating cellular activity.
In addition to providing an ECM for in vivo cellular activity, the amniotic membrane exhibits anti-inflammatory, anti-microbial, anti-scarring, anti-adhesive, angiogenic, and anti- angiogenic properties [1]. The anti-inflammatory effects can be partly attributed to the production of anti-inflammatory factors such as hyaluronic acid, IL-1 receptor agonist, and IL-10 [1]. The expression of anti-microbial molecules like β-defensins and elafin help to explain its antibacterial properties [1]. The membrane may exhibit anti-scarring and anti-adhesive properties due to the down-regulation of transforming growth factor β (TGF-β) and TIMP activity [1]. The amniotic membrane can be angiogenic or anti-angiogenic, depending on the surface it is applied to: it promotes angiogenesis when applied to ischemic organs, and it inhibits angiogenesis in pathological conditions such as cancer [1].
Another useful property that is beneficial for clinical applications is the fact that amniotic membranes do not express HLA markers [8]. Because the amnion must interface with both maternal and embryonic tissue, it must be non-immunogenic for a successful pregnancy. The amniotic membrane acts as an immune privileged tissue containing immunoregulatory factors, such as human leukocyte antigen G (HLA-G), which appears to be an important immunosuppressive factor during pregnancy [8].
The human amniotic membrane provides a relatively ideal 3D scaffold for cellular activity. Three-dimensional tissue- engineering scaffolds are merely analogs of the ECM that is present in all tissues and organs [3]. Studies have shown that cell migration is significantly affected by multiple parameters and properties of the ECM and its microstructure [3]. Tissue engineering of 3D scaffolds with synthetic biomaterials presents a few challenges because they have different mechanical and geometrical properties from the collagen-glycosaminoglycan composition of naturally occurring ECM [3]. Additionally, the influence of the ECM on cellular activity is partly determined by other chemical parameters, like composition, degradation characteristics, and the presence of soluble factors (e.g. growth factors, cytokines) found in the ECM environment.
On the other hand, the use of naturally-derived components for tissue-engineered 3D scaffolds presents another set of risks. Immunogenicity is a very important consideration when creating a biocompatible scaffold for tissue engineering, especially since many ECM components used in creating tissue-engineered scaffolds are of xenogeneic origin [9]. The human amniotic membrane is a very unique biomaterial for clinical treatment because it provides an immune-privileged, allogeneic ECM which bypasses the complications of using xenogeneic tissue [9].
Conclusion
Human amniotic membrane is undoubtedly a very promising biomaterial in medicine due to a myriad of beneficial physical and biochemical properties, the stem cell characteristics of its epithelial and stromal cells, the virtually unlimited availability, and low processing cost. It has a unique combination of characteristics that is not found in other natural or synthetic biocompatible materials which justifies its use in treating a wide variety of clinical conditions. Therefore, the establishment of standardized protocols for the collection and preservation of human amniotic membrane is critical to ensure patient safety and tissue quality. There are a variety of methods for processing and preserving the amnion, with different strengths and weaknesses. It is important to comply with long-term storage regulations, follow appropriate sterilization procedures, and to ensure the results of all appropriate serological testing are negative.
There are reports on the usage of fresh amniotic membrane, but there is a potential risk of disease transmission associated with this procedure [10]. For this reason, preservation of the amnion is critical, but the methods for processing and preserving human amnion affect the properties of the biological material. There are currently various methods for amnion preservation, which include lyophilization, air-drying, hyper-drying, preservation in media, and other chemical methodologies [10]. Additionally, there are a variety of methods used for the de- epithelialization of the amniotic membrane to purely use it as a substrate for cell growth. There is no consensus if this procedure is beneficial or not, but it has been shown that de-epithelialized human amniotic membrane promotes better cell proliferation and differentiation of cultured cells, while intact amniotic membrane can retard migration and differentiation [10]. There are a variety of ways that the human amniotic membrane can be processed, preserved, and stored for clinical use, but the best methods and how they influence the product's mechanism of action have not been fully elucidated.
Disclosures
Dr. Robert S Kellar is an Associate Professor of Practice at Northern Arizona University and serves as the Chief Scientific Officer for Axolotl Biologix in which he has a personal financial interest.
References
- Rocha SCM, Baptista CJM (2015) Biochemical Properties of Amniotic Membrane. In: Mamede A, Botelho M (Eds.), Amniotic Membrane. Springer, Netherlands.
- de Sousa Barros JJ (2015) Embryology and Anatomy of Placental Membranes. In: Mamede A., Botelho M. (Eds,). Amniotic Membrane. Springer, Netherlands.
- Harley BAC, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ (2008) Microarchitecture of Three-Dimensional Scaffolds Influences Cell Migration Behavior via Junction Interactions. Biophysical Journal 95(8): 4013-4024.
- Halim A.S., Bujang-Safawi E., Saad A.Z.M. (2015) Amniotic Membrane in the Treatment of Burns. In: Mamede A, Botelho M (Eds,). Amniotic Membrane. Springer, Netherlands.
- Costa E, Murta JN (2015) Amniotic Membrane in Ophthalmology. In: Mamede A., Botelho M. (eds) Amniotic Membrane. Springer, Netherlands.
- Lo V, Pope E (2009) Amniotic membrane use in dermatology. International Journal of Dermatology 48: 935-940.
- Mermet I, Pottier N, Sainthillier JM, Malugani C, Cairey-Remonnay S, et al. (2007) Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair and Regeneration 15: 459-464.
- Areia AL, Moura P (2015) Amniotic Membrane in Health and Disease: An Obstetrical Perspective. In: Mamede A, Botelho M (Eds,). Amniotic Membrane. Springer, Netherlands.
- Ahmadiani A, Ghanavi J, Jorjani M, Niknejad H, Peirovi H, et al. (2GG8) Properties of the amniotic membrane for potential use in tissue engineering. European cells & materials 15: 88-99.
- Laranjo M (2G15) Preservation of Amniotic Membrane. In: Mamede A, Botelho M (Eds,). Amniotic Membrane. Springer, Netherlands.






























