Concentration-Dependent Effects of Tetrodotoxin in Function of Adult Rat and Rabbit Heart
Yusheng Qu*, BaoXi Gao, Mei Fang and Stefan I McDonough
Integrated Discovery and Safety Pharmacology, CBSS, and Department of Neuroscience, Amgen Inc., Thousand Oaks, CA, USA
Submission: October 27, 2017; Published: November 27, 2017
*Corresponding author: Yusheng Qu, Principal Scientist, Integrated Discovery and Safety Pharmacology, Amgen Inc, Thousand Oaks, California 91320, USA, Tel: 805-447-8093; Email: yqu@amgen.com
How to cite this article: Qu, Y., Gao, BX., Fang, M., McDonough, SI. Concentration-Dependent Effects of Tetrodotoxin in Function of Adult Rat and Rabbit Heart. J of Pharmacol & Clin Res. 2017; 4(2): 555636. DOI: 10.19080/JPCR.2017.04.555636
Abstract
Tetrodotoxin (TTX) has differential effects on voltage-gated sodium channels and has been used routinely in characterizing functional contributions of different Na channel isoforms. In whole heart, however, it never has been tested at concentrations above 200 nM, leaving some ambiguity as to the roles of TTX-sensitive and TTX-resistant sodium channels to excitation and contractility of intact heart, We have used Lang end or ff perfusion of TTX to investigate the concentration dependence of its effects in isolated adult rat and rabbit hearts and compared to potency in vitro. In rat heart, 50 nM TTX, sufficient to block much TTX-S current in vitro, had no effects on electrocardiogram (ECG) or on left ventricular contractility (LVC). 100 nM TTX gave a small reduction in heart rate (HR) and in LVC but no discernable change in ECG intervals, and3 μ M TTX produced significant decrease in HR and LVC and prolongation of PR and QRS intervals. In adult rabbit heart, 100 nM TTX had no detectable effect on any parameters and 3 μM TTX gave a 40% prolongation of PR and QRS intervals but only small decreases in HR and in LVC.
Taken with existing data on all species, results suggest a rather complex dependence of cardiac parameters on different sodium channels. Results here suggest that in adult rabbit and rat heart TTX-R channels play a major role in excitation and conduction, whereas TTX-S channels do not contribute significantly to excitation and conduction, and that there are species differences between rat and rabbit in the isoforms or the density of sodium channels governing HR and LVC.
Keywords: Tetrodotoxin; Sodium Channels; Isolated hearts; Rat; Rabbit; ECG; QRS intervals
Abbreviations: HR: Heart Rate; ECG: Electro Cardio Gram; LVC: Left Ventricular Contractility; TTX-S: Tetrodotoxin-Sensitive; TTX-R: Tetrodotoxin- Resistant; LVDP: LV diastolic pressure; CPP: Coronary perfusion pressure; CF: Coronary flow
Introduction
Voltage-gated sodium channels (Navs) govern many aspects of cardiac excitability, including the depolarization of atrial and ventricular myocytes and the propagation of excitation from sinoatrial node (SAN). There are nine isoforms of sodium channel alpha subunits, Nav1.1 through Nav1.9, and these show differences in tissue distribution, biophysical properties, physiological roles, and pharmacology [1,2]. Pharmacologically, sodium channels can be grouped into tetrodotoxin-sensitive (TTX-S) channels Nav1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.6, and Nav1.7, which are inhibited by TTX with IC50 tens of nanomolar, and tetrodotoxin-resistant (TTX-R) channels Nav1.5, Nav1.8, and Nav1.9, which are inhibited by micromolar or higher concentrations of TTX [3].
Several sodium channels are expressed in the heart and show differential patterns of expression, and recent work suggests isoform-specific physiological roles. Nav1.5 clearly is the major cardiac sodium channel and encodes much or most of the current recorded from individual cardiomyocytes. Based on data from clinical genetics, Nav1.5 shapes cardiac- wide excitation as manifested in PR, QRS, and QT intervals and in pacemaking [4]. Roles for Nav1.5 at the whole-heart level have not been tested pharmacologically, however, likely due to the experimental requirement for large amounts of TTX. Polymorphisms in SCN10A associated with cardiac function suggest the Nav1.8 locus also may affect cardiac function, either directly via Nav1.8 or indirectly via Nav1.5 [5-7]. Immunofluorescence, transcript localization, and some functional measurements at the cellular and whole-heart level also suggest a role for TTX-S sodium channels in cardiac function. Most measurements have been made in mouse, however, and different species express quite different combinations of cardiac ion channels. Mechanistic understanding of the roles of different sodium channels among species is also important from the drug discovery point of view, to define cardiac selectivity hurdles and appropriate preclinical species for safety testing. Rabbit in particular is a common species for physiological investigations and preclinical toxicology testing, and there is little detailed information on the functional role of sodium channel subtypes in rabbit.
We have tested directly the effects of increasing concentrations of TTX on excitation, conduction, and contractility parameters of the whole heart, recorded in the Langendorff preparation from adult rat and rabbit hearts. In both species we find prolongation of the QRS intervals correlates with inhibition of TTX-R channels. In rat we find a major role for TTX-R channels in heart rate (HR) and left ventricular contractility (LVC). By contrast, in rabbit we find only a very modest contribution of TTX-R channels to HR and to LVC. In neither rat nor rabbit do we find a significant functional role for TTX-S channels. We interpret this as more likely to reflect underlying biology than poor tissue distribution, although there is no direct measure of target occupancy in the Langendorff preparation. Low TTX concentrations sufficient to inhibit fully TTX-S channels in vitro had small or no effect on cardiac contractility parameters, whereas effective concentrations in the Langendorff preparation were consistent with concentrations required to inhibit TTX-R channels in vitro. Supporting this, in testing compounds with a variety of physicochemical properties, we find no evidence for large disconnects between potency in vitro and potency in the Langendorff preparation.
Methods
Electrophysiological Recording of hNav1.5 current
HEK 293 cells stably-transfected with human Nav1.5 (hNav1.5) cDNA were licensed from Millipore (Billerica, MA). Whole cell currents were recorded with the Patch Xpress 7000A planar patch clamp automated electrophysiology system (Molecular Devices, Sunnyvale, CA) using manufacturer's procedures. Extracellular solution contained, in mM: NaCl 70, N-methyl-D-glucamine 67, KCl 4.0, CaCl2 1.8, MgCl2 1, HEPES 10, glucose 10, pH= 7.40 with HCl; intracellular solution contained, in mM: CsF 130, NaCl 10, EGTA 10, MgCl2, HEPES 10, pH= 7.20 with CsOH. When a quality whole-cell recording was established, cells were washed for 2 minutes, followed by applying control vehicle for 5 minutes. Then control plus each concentration of test article were applied for 5 minutes (3 additions for each concentration at 1 minute intervals). A standardized step protocol was used to elicit ionic current through the hNav1.5 sodium channel.
Cells were held at -80 mV. Onset and steady state block of hNav1.5 sodium current was measured using a pulse pattern with fixed amplitudes (conditioning prepulse: -120 mV for 50 ms; depolarizing test step to -30 mV for 20 ms). The stimulation paradigm was repeated once every 10 s (0.1 Hz). Currents were filtered at 3 kHz and acquired at 10 kHz in episodic mode. Experiments were conducted at room temperature (20C - 22C). Potency of TTX on hNav1.7 and hNav1.4 expressed in 293 cells was measured with manual patch-clamp electrophysiology, with external solution (in mM): NaCl 140, HEPES 10, glucose 11, KCl 5, CaCl2, MgCl2 1.1, pH 7.4 with NaOH; and internal solution (in mM): CsF 75, CsCl 62.5, MgCl2.5, HEPES 10, EGTA 5, pH 7.25 with CsOH. Currents were recorded from a steady holding voltage of -140 mV and evoked with a 10 ms pulse to -20 mV delivered every 5 seconds. For solution exchange, cells were lifted off the bottom of the dish with the patch pipette and positioned directly in front of a micropipette connected to a solution exchange manifold. Currents were digitized at 10 kHz and filtered at 1 kHz and analyzed offline with pCLAMP software. Experiments were conducted at room temperature (20-22oC).
Isolated Rat Heart and Isolated Rabbit Heart Using Langendorff Perfusion
Female New Zealand White rabbits (2.5-3.5 kg) and male Sprague Dawley rats (300 - 500g) were obtained from Harlan Laboratories, Inc. (Placentia, CA, USA) and were cared for in accordance to the Guide for the Care and Use of Laboratory Animals, 8th Edition (NRC 2011). Rabbits and rats were singlehoused at an AAALAC internationally-accredited facility. All rabbit and rat experiments were conducted in compliance with the Amgen Institutional Animal Care and Use Committee and USDA regulations. The procedure was similar to that described previously [8]. Briefly, rabbits were anesthetized with pentobarbital (50 mg/kg) by ear vein injection. Rats were anesthetized with pentobarbital (50 mg/kg) by intraperitoneal injection.
Following the induction of a surgical plane of anesthesia, the heart was rapidly removed, which resulted in the humane euthanasia of the animal. The heart was cannulated via the aorta and perfused according to the Langendorff technique with a modified Krebs-Henseleit solution composed of (in mM): 120 NaCl, 4.7 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 11.1 Glucose, 2 Na-pyruvate, 1.8 CaCl2 and bubbled with O2/CO2 (95%/5%). Two lead ECGs were recorded with flexible unipolar electrodes (Harvard Apparatus, Holliston, MA) placed on the heart, one over the epicardium of ventricles and the other over the epicardium of the left atria. For measuring left ventricular contractility, a metal cannula with a rubber balloon on the tip connected to a pressure transducer was inserted through the mitral orifice so that the balloon resided within the left ventricle (LV).
For hemodynamic measurements, the latex balloon in the LV was expanded with water to achieve a LV diastolic pressure (LVDP) of approximately 5-10 mm Hg. Once the end diastolic pressure was stabilized at the baseline, it was not adjusted during the course of the experiment. Coronary perfusion pressure (CPP) was measured with a pressure transducer connected to the aortic block. Coronary flow (CF) was measured with an inline transonic flow probe (Harvard Apparatus, Holliston, MA) inserted in the aortic block. Control measurements of all physiological variables were made for at least 45 minutes during the perfusion with Krebs solution. Hearts were then perfused (20 min per dose) with escalating concentrations of TTX. All studies were conducted under constant perfusion pressure and at 37C. LVP, CPP, and ECG were recorded with the Notocord HEM data capture system and EMKA Auto software. Six hearts were tested at each concentration of TTX.
Data Acquisition and Analysis
For the IRH assay, LVP, CPP, and CF were automatically analyzed with Notocord, and ECG were analyzed with EMKA Auto software. For heart rate correction of the QT interval, the Fridericia equation (QTcF=QT/RR1/3) was used. Values from each individual heart were pooled to determine an average for each variable at individual concentrations. All numeric values derived in these studies are presented as mean ± S.E.M
Results
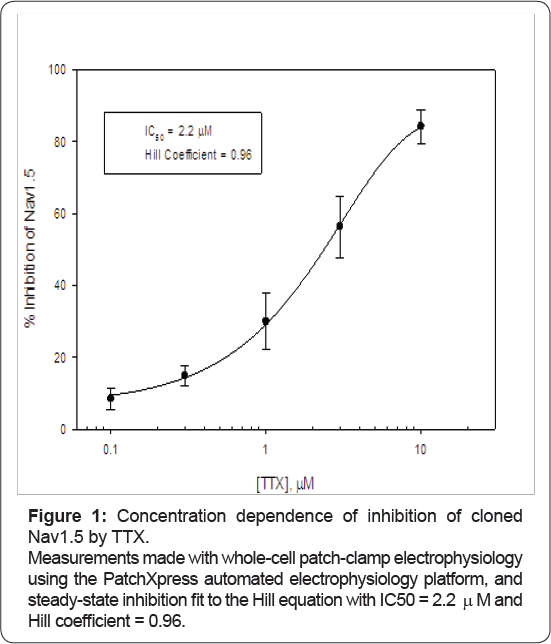
To verify that preparations and reagents in our hands were consistent with previous reports, TTX was checked in vitro for its potency on cloned human Nav1.5, a TTX-R sodium channel, and on cloned human Nav1.7 and Nav1.4, TTX-S sodium channels, with whole-cell patch clamp electrophysiology. Potency in our hands was fit well by 1:1 binding with IC50 = 2.2 μM for Nav1.5 (Figure 1). We also measured potency on two TTX-S channels and recorded IC50 of 54 nM for Nav1.7 and 24 nM for Nav1.4, in agreement with previous measurements [9-13] and with previous literature that TTX has the same potency and molecular binding site on Nav1.1, 1.2, 1.3, 1.4, 1.6, and 1.7 isoforms [1,14]. Data are consistent with previous results showing the heart isoform of Nav1.5 is unaffected by 100 nM TTX [3,15]. Block was reversible, and potency (assessed on Nav1.7 and Nav1.4) was similar measured on wholly noninactivated or partially-inactivated channels, consistent with the known TTX mechanism of pore block [2,16-18].
With these potencies in mind, we then perfused ascending concentrations of TTX through adult rabbit and adult rat heart. Hearts in the Langendorff preparation were equilibrated for 30 to 60 minutes until ECG parameters stabilized and then were perfused retrogradely with 100 nM, 1 μM and 3 μM TTX. 50 nM TTX was tested in adult rat heart only at single-point, to determine better any responsiveness of rat heart to inhibition of TTX-S channels. Extrapolating from in vitro measurements, 50 nM and 100 nM should inhibit approximately 70% and 80% of TTX-S channels and < 10% of TTX-R channels; 1 μM should inhibit essentially all TTX-S channels and ~ 30% of TTX-R channels; and the highest concentration of 3 μM should inhibit all TTX-S channels and ~ 60% of TTX-R channels (Figure 1). (Materials availability precluded testing 100% inhibition of TTX-R channels in whole heart with tetrodotoxin.)
ECG was recorded from unpaced, spontaneously beating hearts for a total of 20 minutes from each concentration, and parameters including HR, PR interval, QRS duration, and QT interval were measured continuously. From these parameters QTc interval (QT interval corrected for heart rate), JT interval (QT - QRS, the duration of ventricular repolarization), and JTc (JT corrected for heart rate) were calculated. Contractility parameters were recorded in parallel: left ventricular systolic and diastolic pressure (LVSP and LVDP) and calculated developed pressure (LVDevP = LVSP - LVDP), maximal rates of pressure increase and decrease (dP/dtmax and dP/dtmin), and coronary flow (CF) and coronary perfusion pressure (CPP).
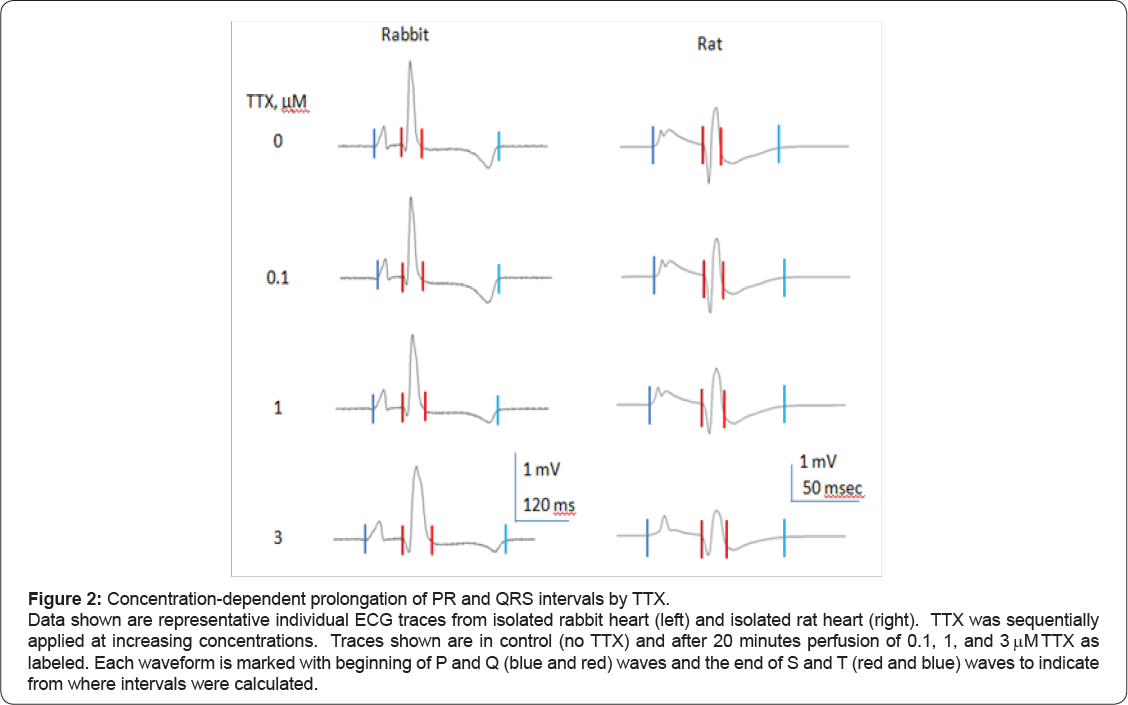
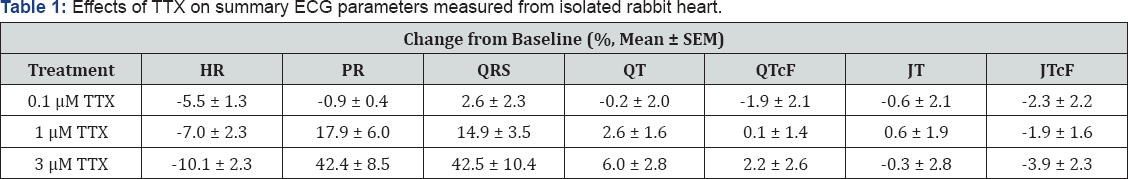
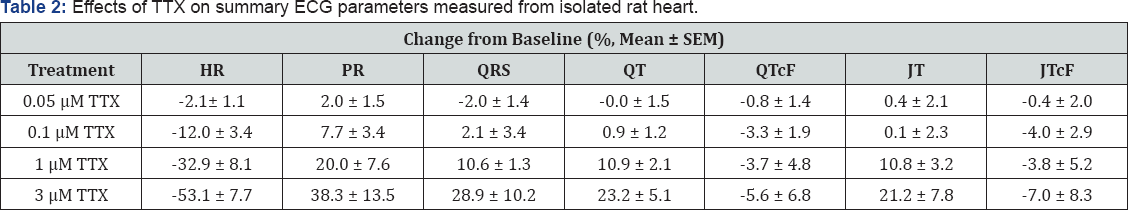
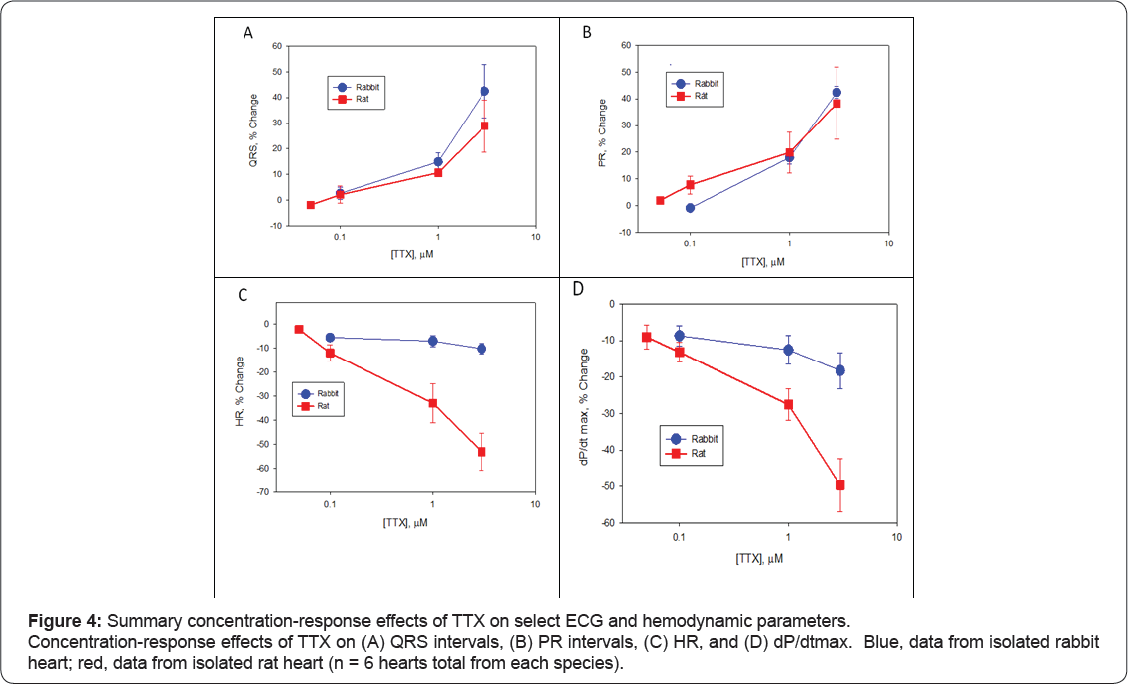
Figure 2 shows representative raw traces from a single ECG cycle from rabbit heart (left) and rat heart (right), and Figure 3 shows representative raw traces from multiple ECG cycles from rabbit heart (left) and rat heart (right). Each trace was recorded from a single heart in control and at steady-state in response to sequential perfusion of 0.1, 1, and 3 μ TTX. Figure 4 shows graphically summary data as percent change in baseline for changes in QRS and PR intervals, HR, and LVP, and Tables 1-4 show summary results of all ECG and LVP parameters respectively (n = 6 hearts for both species). TTX perfusion did affect ECG and LVP parameters, with qualitative differences between rat and rabbit. In both species, the PR and QRS intervals were prolonged by 1 (μand by 3 (μTTX, and little or not at all by 100 nM TTX, suggesting a role for TTX-R channels and not for TTX-S channels. The small prolongation by 100 nM TTX of PR intervals in rat heart was examined further by testing 50 nM TTX, which had no effect, suggesting that the effect of 100 nM might have been from a small effect on TTX-R channels rather than from inhibition of TTX-S channels. These data correspond to existing preclinical, clinical, and genetic data showing that Nav1.5 governs propagation of cardiac excitation, likely through expression at intercalated disks of cardiomyocytes [19-24]. Concentrations of TTX required prolong QRS intervals in both concentration required to inhibit Nav1.5 in vitro (Figures 4 & 5). species were fairly consistent with each other and with the concentration required to inhibit Nav1.5 in vitro (Figures 4 & 5).

Shown are mean as percent change from baseline and standard error of the mean for n=6 hearts.
Shown are mean as percent change from baseline and standard error of the mean for n=6 hearts.
Shown are mean percent change from baseline and standard error of the mean for n=6 hearts.
Shown are mean percent change from baseline and standard error of the mean for n = 6 hearts.
Heart rate was slowed by TTX in a species-specific manner (Figures 3 & 4C). Whereas in rabbit all concentrations had no or small effect on spontaneous heart rate, in rat TTX produced a strong, dose-dependent decrease (Figures 3 & 4C) that at the two highest concentrations was statistically different from rabbit. The average decrease in heart rate induced by TTX for 100 nM was 5.5% ± 1.3% (rabbit) and 12.0% ± 3.4% (rat) (p = 0.11); for 1 μ was 7.0% ± 2.3% (rabbit) and 32.9% ± 8.1% (rat) (p = 0.012); and for 3 was 10.2% ± 2.3% (rabbit) and 53.1% ± 7.7% (rat) (p = 0.0003) (all values mean ± S.E.M., n = 6, rabbit vs. rat, unpaired t-test). Given this strong role of TTX-R channels, it is somewhat more difficult to say definitively whether TTX-S channels in rat also have a small effect on heart rate and contractility, since 100 nM TTX should inhibit 5% to 10% of TTX-R current.
The lack of effect of 50 nM TTX on rat suggests either that TTX-S channels do not themselves play a detectable role, or that very high receptor occupancy of TTX-S channels is needed to show a functional effect. Contractility parameters showed a corresponding species divergence (Figure 4D). TTX had only quite small effects on contractility parameters in rabbit heart, with unclear dose dependence (Table 3). Consistent with this, there was a corresponding small decrease in dP/dt min, systolic pressure, and developed pressure. Rat heart showed much larger reductions in dP/dt max (Table 4) as well as dP/dt min, systolic pressure, developed pressure, with reduction apparent at 1 μ TTX and larger at 3 μTTX, consistent with inhibition of Nav1.5. Again, we infer a role for TTX-R channels, most likely Nav1.5, in the contractility of rat heart that is not apparent in rabbit heart with these concentrations of TTX. TTX-S channels apparently play little or minimal role in the contractility parameters measured in either rat or in rabbit.
In comparing the potency of TTX in intact heart to its potency in vitro, the assumption is that the concentration of TTX in the perfusate equaled the effective concentration at the channel, including deep within the tissue. The quantitative correlation between the concentration dependence of TTX prolongation of QRS waves of intact heart and the inhibition of Nav1.5 in vitro is consistent with this (Figure 5), although this is to some extent a circular argument, since despite its known links to cardiac conduction determined genetically, in this paper sensitivity to micromolar TTX concentrations is used to infer involvement of Nav1.5. An alternative is that TTX did not diffuse well in intact heart, and so effects of 1 μ M and 3 μ M TTX could have been due to specific inhibition of TTX-S channels and not TTX-R by a smaller effective tissue concentration than applied in the perfusate. No such concentration shift, however, was seen in our previous experiments with the 36-mer peptide BeKml [8], a much larger molecule that might be presumed to have poorer tissue access than TTX.

In rabbit heart, 10 nM BeKml caused statistically significant increases in the QTc interval consistent with the IC50 of 12 nM on the hERG potassium channel measured in vitro. Likewise, the class 1C anti-arrhythmic drug flecainide (smaller than TTX but like TTX a polar, soluble, and poorly protein-bound molecule), prolonged the QRS duration at concentrations one micromolar and above, comparable to its in vitro IC50 on Nav1.5 [24]. Finally, to search for physicochemical conditions with which we might uncover a mismatch between Nav1.5 potency in vitro and concentration to prolong QRS wave in intact heart, we tested "compound 52," N-(2-methyl-3-((4-(4-((4-(trifluoromethoxy) benzyl)oxy)-1-piperidinyl)-1,3,5-triazine-2-l)amino)phenyl) acetamide [22].
Whereas flecainide, TTX, and BeKml might be expected to have poor penetration within tissue membranes due to size and / or polarity, "compound 52" is quite hydrophobic and much more highly protein-bound than TTX (97% vs 20%, [23]) and if anything might be expected to accumulate in intact tissue. As with the other sodium channel blockers, however, compound 52 prolonged the QRS interval in rabbit heart in a dose-dependent manner, 13% ± 5.5% at 0.3 μ M and 25.8% ± 7.4% at 1 μ M (mean ± S.E.M., n = 4), potency qute comparable to its IC50 of 1.1 μM on expressed cloned hNav1.5 in vitro. Correspondence between in vitro IC50 and the effect on QRS interval in intact heart for hydrophobic and hydrophilic small molecules and for a peptide, and for TTX itself, argues that there is no major concentration gradient of TTX within intact heart that might confound interpretation.
Discussion
The dominant role of the TTX-R Nav1.5 channels in cardiac excitation has long been known, through transcript and immunofluorescence-based protein localization, cellular physiology, transgenic mice, and increasingly clinical genetics. Here we complement these data with functional testing of the entire heart in response to a pharmacological antagonist, tetrodotoxin, that unambiguously inhibits only sodium channels. This is the first study that examines effect of TTX above 200 nM in isolated intact hearts. Within the sodium channel family, the approximately 100-fold differences in tetrodotoxin sensitivity between isoforms, sensitivity understood at the molecular level, enables us to make inferences on the roles of TTX-S and TTX-R sodium channels. In some cases our data can be well understood within the existing framework of sodium channel expression, and in other cases not, suggesting either differences among experimental approaches or mechanistic biology that has yet to emerge.
Data here on the role of TTX-R sodium channels in the QRS wave in rat and in rabbit are consistent with the location and function of TTX-R Nav1.5 in a variety of species and with the role of Nav1.5 in human, as deduced by clinical genetic studies [2532] (for review see [4]). Across species Nav1.5 expression is the strongest of any sodium channel in heart; the majority of current from patch-clamped atrial and ventricular myocytes is TTX-R; and immunofluorescence studies show localization of Navl.5 at intercalated disks in mouse and rat [19,33-37] (although this is subtle in rabbit [38]), a position ideally situated to govern surface conduction and inter-myocyte electrical propagation. Accordingly, we ascribe the effects shown here of one and three micromolar TTX on prolongation of the QRS wave, in rat and in rabbit, to inhibition of Nav1.5, given that Nav1.8 and Nav1.9 are insensitive to these concentrations [39-41]. Note also that SCN10A knockout mice showed unchanged QRS duration and shortened PR intervals [5], opposite to the results reported here with 3 μMTTX, consistent with a role for Nav1.5 and inconsistent with a role for Nav1.8.
In our hands, block of TTX-R channels had little to no effect on HR and LVP in rabbit. This also is consistent with expression data showing little if any Nav1.5 transcripts in the center of rabbit SAN [42] and no voltage-gated sodium currents in rabbit SAN [43,44]. By contrast, in rat, our data from intact heart show no effects of 50 nM TTX, small effects of 100 nM TTX, and profound slowing of HR by 1 μM and 3 μM TTX, suggesting a strong role for Nav1.5 and no detectable role for TTX-S channels alone in pace making. (The very small effects of 100 nM TTX on rat HR we ascribe to small inhibition of Nav1.5, since 50 nM TTX had no effect and since higher TTX clearly showed a major role for Nav1.5.) These results in rat are not easy to reconcile with transcript expression. Studies of rat SA node have found strong expression of TTX-S Nav1.1 and Nav1.6 transcripts but little expression of Nav1.5 [45].
Data from adult mouse heart also show expression of TTX-S isoforms in SAN, and small effects of 100 nM TTX on spontaneous HR [46]. Setting aside for now technical concerns about probe specificity for channel localization, TTX access to intact tissue for functional measurements, and cryptic channels blocked by micromolar TTX, it is difficult to reconcile these expression patterns with our functional data. One possibility might be that in rat, Nav1.5 inhibition affects propagation from the SAN and thereby enables a non-primary pacemaker driven by Nav1.5, but this is in conflict with the consistent presence of P waves and prolongation of the PR intervals we observed.
Similarly, some aspects of our results on the role of different sodium channels in myocyte contractility are consistent with tissue expression, and some are not. Strong reduction in LVP in rats upon TTX-R channel inhibition as measured here is consistent with the dominant expression of Nav1.5 in rat ventricle, and it seems fairly straightforward that inhibition of excitability also would inhibit downstream contractility. Nav1.5, however, is also strongly expressed in rabbit ventricle, and we did not see a corresponding reduction in rabbit LVC with increasing TTX concentrations. Apart from invoking unknown channel distributions or tissue structures, one possibility is that Nav1.5 is present at higher density in rabbit ventricular myocytes than in rat ventricular myocytes, and so requires greater fractional inhibition to affect function. Strong nonlinearity between Nav1.5 inhibition and function (upstroke velocity) has been described in rabbit Purkinje fibers, such that three (MTTX eliminates over 80% of sodium current under voltage clamp yet reduces action potential upstroke velocity only ~ 25% [47,48]. Especially given the additional downstream steps between depolarization and calcium-induced contraction it seems reasonable to expect strong nonlinearity between Nav1.5 inhibition and contraction.
A role for TTX-S sodium channels in contractility is attracting increasing attention due to localization in ventricular myocytes of TTX-S isoform transcripts and protein, particularly at T-tubule membranes, and to presence of TTX-S sodium currents [12,19,21,35,49-52]. Small reductions in contractility in whole heart in response to 100 nM and 200 nM TTX were observed in mouse heart and in guinea pig heart [12], similar to that observed here in rat heart. These results at first might seem at odds with our suggestion that in both adult rabbit and adult rat heart, inhibition of TTX-S channels had minimal effect on contractility. (As with heart rate, we ascribe the small effects of 100 nM TTX on rat LVC to the start of a concentration- dependent large effect via TTX-R channel inhibition.) Most work on cardiac TTX-S channels, however, has taken place in mouse heart, and differences in ion channel and sodium channel compositions [53] are common among species and at different stages of development. Most comparable to our work, in ventricular myocytes from rat heart Brette and Orchard found TTX-S current preferentially in T-tubules, yet found no effects of 200 nM TTX on cytoplasmic Ca2+ or on myocyte contractility evoked by field stimulation [51], in agreement with our result from whole rat heart. In ventricular myocytes from rabbit a similar study reported a decline in cytoplasmic Ca2+ in response to 100 nM TTX, possibly mediated by TTX-S sodium currents priming sodium-calcium exchanger in reverse mode [54], but contractility was not assessed.
TTX-S sodium channels seem unlikely to play a major role in human heart, since few transcripts are expressed, polymorphisms associated with neuronal disease do not affect the cardiac system, and TTX poisoning does not impair cardiac excitation [55], and our results add to the picture that TTX-S sodium channels may play less or little role in species with larger hearts and slower heart rates. Our results are the first to our knowledge to report the effects of high concentrations of TTX (> 200 nM) on intact heart of any species, confirming a strong role for TTX-R sodium channels, and so will be useful as distribution and functional roles of sodium channels are explored in different species.
Sodium channel pharmacology is an intensely active area. Associations of neuronal sodium channels with various path physiological processes including pain, itch, multiple sclerosis, and epilepsy have led to some success in engineering small molecule Nav inhibitors with isoform selectivity [56-58], and additional efforts show the potential for treating disease by introducing nonselective sodium channel inhibitors into neurons via the pore of TRP channels [59-62]. In the flow path to develop such drugs that govern neuronal excitability, cardiac safety is a main toxicology obstacle, and results here suggest that TTX-S sodium channels are not a cardiac safety liability despite their presence in tissues of preclinical species. Results here also show that HR and LVC in rabbit heart is a less sensitive readout of inhibition of TTX-R current than in rat heart. PR and QRS intervals, however, demonstrate the same sensitivity to TTX in both species. Accordingly, for identifying risks associated with Nav1.5 channel inhibition by test molecules, prolongation of PR and QRS intervals are the preferred end parameters [63].
Acknowledgement
This study would not have been possible without the support of Dr. Hugo M. Vargas. We also thank Dr. Vargas for his review and comments of this manuscript.
References
- Catterall WA (2012) Voltage-gated sodium channels at 60: structure, function and pathophysiology. The Journal of physiology 590(11): 2577-2589.
- Catterall WA, Goldin AL, Waxman SG (2005) International Union of Pharmacology. XLVII Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacological reviews 57(4): 397-409.
- Goldin AL (2001) Resurgence of sodium channel research. Annual review of physiology 63: 871-894.
- Wilde AA, Brugada R Phenotypical (2011) manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circulation research 108(7): 884-897.
- Chambers JC, Zhao J, Terracciano CM, Bezzina CR, Zhang W, et al. (2010) Genetic variation in SCN10A influences cardiac conduction. Nature genetics 42(2): 149-152.
- Park DS, Fishman GI (2014) Nav-igating through a complex landscape: SCN10A and cardiac conduction. The Journal of clinical investigation 124(4): 1460-1462.
- van den Boogaard M, Smemo S, Burnicka-Turek O, Arnolds DE, van de Werken HJ, et al.(2014) A common genetic variant within SCN10A modulates cardiac SCN5A expression. The Journal of clinical investigation 124(4): 1844-1852.
- Qu Y, Fang M, Gao B, Chui RW, Vargas HM (2011) BeKm-1 a peptide inhibitor of human ether-a-go-go-related gene potassium currents, prolongs QTc intervals in isolated rabbit heart. The Journal of pharmacology and experimental therapeutics 337(1): 2-8.
- Antoni H, Bocker D, Eickhorn R (1988) Sodium current kinetics in intact rat papillary muscle: measurements with the loose-patch-clamp technique. The Journal of physiology 406: 199-213.
- Doyle DD, Guo Y, Lustig SL, Satin J, Rogart RB, et al. (1933) Divalent cation competition with [3H]saxitoxin binding to tetrodotoxin- resistant and -sensitive sodium channels. A two-site structural model of ion/toxin interaction. The Journal of general physiology 101(2): 153-182.
- Guo XT, Uehara A, Ravindran A, Bryant SH, Hall S, et al. (1987) Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle. Biochemistry 26(24): 7546-7556.
- Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, et al. (2002) An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proceedings of the National Academy of Sciences of the United States of America 99(6): 4073-4078.
- Muramatsu H, Zou AR, Berkowitz GA, Nathan RD(1996) Characterization of a TTX-sensitive Na+ current in pacemaker cells isolated from rabbit sinoatrial node. The American journal of physiology. 270(6\2): H2108-H2119.
- Catterall WA (1980) Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annual review of pharmacology and toxicology 20: 15-43.
- Cribbs LL, Satin J, Fozzard HA, Rogart RB (1990) Functional expression of the rat heart I Na+ channel isoform. Demonstration of properties characteristic of native cardiac Na+ channels. FEBS letters 275(1-2): 195-200.
- Fozzard HA, Hanck DA (1996) Structure and function of voltage- dependent sodium channels: comparison of brain II and cardiac isoforms. Physiological reviews 76(3): 887-926.
- Gellens ME, George AL, Jr Chen LQ, Chahine M, Horn R, et al. (1992) Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proceedings of the National Academy of Sciences of the United States of America 89(2): 554-558.
- Satin J, Kyle JW, Chen M, Bell P, Cribbs LL, et al. (1992) A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science 256(5060): 1202-1205.
- Haufe V, Camacho JA, Dumaine R, Gunther B, Bollensdorff C, et al. (2005) Expression pattern of neuronal and skeletal muscle voltage- gated Na+ channels in the developing mouse heart. The Journal of physiology 564(3): 683-696.
- Kucera JP, Rohr S, Rudy Y (2002) Localization of sodium channels in intercalated disks modulates cardiac conduction. Circulation research 91(12): 1176-1182.
- Westenbroek RE, Bischoff S, Fu Y, Maier SK, Catterall WA, et al. (2013) Localization of sodium channel subtypes in mouse ventricular myocytes using quantitative immunocytochemistry. Journal of molecular and cellular cardiology 64: 69-78.
- Bregman H, Berry L, Buchanan JL, Chen A, Du B, et al. (2011) Identification of a potent, state-dependent inhibitor of Nav1.7 with oral efficacy in the formalin model of persistent pain. Journal of medicinal chemistry 54(13): 4427-4445.
- Matsumoto T, Tanuma D, Tsutsumi K, Jeon JK, Ishizaki S, et al. (2010) Plasma protein binding of tetrodotoxin in the marine puffer fish Takifugu rubripes. Toxicon: official journal of the International Society on Toxinology 55(2-3): 415-420.
- Qu Y, Gao B, Fang M, Vargas HM (2013) Human embryonic stem cell derived cardiac myocytes detect hERG-mediated repolarization effects, but not Nav1.5 induced depolarization delay. Journal of pharmacological and toxicological methods 68(1): 74-81.
- Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, et al. (2008) Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation 117(15): 1927-1935.
- Ellinor PT, Nam EG, Shea MA, Milan DJ, Ruskin JN, et al. (2008) Cardiac sodium channel mutation in atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society 5(1): 99-105.
- Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, et al. (2002) Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. The Journal of clinical investigation 110(8): 1201-1209.
- Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, et al. (2010) An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart rhythm: the official journal of the Heart Rhythm Society 7(1): 33-46.
- Kyndt F, Probst V, Potet F, Demolombe S, Chevallier JC, et al. (2001) Novel SCN5A mutation leading either to isolated cardiac conduction defect or Brugada syndrome in a large French family. Circulation 104(25): 3081-3086.
- Makiyama T, Akao M, Shizuta S, Doi T, Nishiyama K, et al. (2008) A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. Journal of the American College of Cardiology 52(16): 1326-1334.
- Schott JJ, Alshinawi C, Kyndt F, Probst V, Hoorntje TM, et al. (1999) Cardiac conduction defects associate with mutations in SCN5A. Nature genetics 23(1): 20-21.
- Smits JP, Koopmann TT, Wilders R, Veldkamp MW, Opthof T, et al. (2005) A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. Journal of molecular and cellular cardiology 38(6): 969-981.
- Cohen SA (1996) Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation 94(12): 3083-3086.
- Dominguez JN, de la Rosa A, Navarro F, Franco D, Aranega AE, et al. (2008) Tissue distribution and subcellular localization of the cardiac sodium channel during mouse heart development. Cardiovascular research 78(1): 45-52.
- Maier SK, Westenbroek RE, Curtis R, Scheuer T, Catterall WA, et al. (2004) Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation 109(11): 1421-1427.
- Malhotra JD, Thyagarajan V, Chen C, Isom LL (2004) Tyrosine- phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. The Journal of biological chemistry 279(39): 40748-40754.
- Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, et al. (2011) SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circulation research 108(3): 294-304.
- Gershome C, Lin E, Kashihara H, Hove-Madsen L, Tibbits G, et al. (2011) Colocalization of voltage-gated Na+ channels with the Na+ /Ca2 + exchanger in rabbit cardiomyocytes during development. American journal of physiology Heart and circulatory physiology 300(1): H300-H311.
- Akopian AN, Sivilotti L, Wood JN (1996) A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379(6562): 257-262.
- Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, et al. (1999) A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. The Journal of neuroscience the official journal of the Society for Neuroscience 19(24): RC43.
- Dib-Hajj S, Black JA, Cummins TR, Waxman SG, (2002) A sodium channel with unique properties. Trends in neurosciences 25(5): 253259.
- Tellez JO, Dobrzynski H, Greener ID, Graham GM, Laing E, et al. (2006) Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit. Circulation research 99(12): 1384-1393.
- Baruscotti M, DiFrancesco D, Robinson RB (1996) A TTX-sensitive inward sodium current contributes to spontaneous activity in newborn rabbit sino-atrial node cells. The Journal of physiology 492 (Pt 1): 21-30.
- Baruscotti M, Westenbroek R, Catterall WA, DiFrancesco D, Robinson RB, et al. (1997). The newborn rabbit sino-atrial node expresses a neuronal type I-like Na+ channel. The Journal of physiology 498( Pt 3): 641-648.
- Du Y, Huang X, Wang T, Han K, Zhang J, et al. (2007) Down regulation of neuronal sodium channel subunits Nav1.1 and Nav1.6 in the sinoatrial node from volume-overloaded heart failure rat. European journal of physiology 454(3): 451-459.
- Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, et al. (2003) An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proceedings of the National Academy of Sciences of the United States of America 100(6): 3507-3512.
- Cohen CJ, Bean BP, Tsien RW (1984) Maximal upstroke velocity as an index of available sodium conductance. Comparison of maximal upstroke velocity and voltage clamp measurements of sodium current in rabbit Purkinje fibers. Circulation research (6): 636-651.
- Bean BP, Cohen CJ, Tsien RW (1982) Block of cardiac sodium channels by tetrodotoxin and lidocaine: Sodium current and Vmax experiments. In: Paes de Carvalho A, Hoffman BF, Lieberman M (Eds.), Normal and Abnormal Conduction in Heart, Futurea, New York, USA, pp. 189-209.
- Brette F, Orchard CH (2006) Density and sub-cellular distribution of cardiac and neuronal sodium channel isoforms in rat ventricular myocytes. Biochemical and biophysical research communications 348(3): 1163-1166.
- Kaufmann SG, Westenbroek RE, Zechner C, Maass AH, Bischoff S, et al. (2010) Functional protein expression of multiple sodium channel alpha and beta subunit isoforms in neonatal cardiomyocytes. Journal of molecular and cellular cardiology 48(1): 261-269.
- Brette F, Orchard CH. (2006) No apparent requirement for neuronal sodium channels in excitation-contraction coupling in rat ventricular myocytes. Circulation research 98(5): 667-674.
- Verkerk AO, van Ginneken AC, van Veen TA, Tan HL, et al. (2007) Effects of heart failure on brain-type Na+ channels in rabbit ventricular myocytes. Europace European pacing arrhythmias and cardiac electrophysiology journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 9(8): 571-577.
- Blechschmidt S, Haufe V, Benndorf K, Zimmer T (2009) Voltage-gated Na+ channel transcript patterns in the mammalian heart are species- dependent. Progress in biophysics and molecular biology 98(2-3): 309-318.
- Torres NS, Larbig R, Rock A, Goldhaber JI, Bridge JH (2010) Na+ currents are required for efficient excitation-contraction coupling in rabbit ventricular myocytes a possible contribution of neuronal Na+ channels. The Journal of physiology 588(Pt 21): 4249-4260.
- Zimmer T, Haufe V, Blechschmidt S (2014) Voltage-gated sodium channels in the mammalian heart. Global cardiology science & practice 2014(4): 449-463.
- Bagal SK, Chapman ML, Marron BE, Primer R, Storer IR, et al. (2014) Recent progress in sodium channel modulators for pain. Bioorganic and Medicinal Chemistry Letters.
- Goldberg YP, Price N, Namdari R, Cohen CJ, Lamers MH, et al. (2012) Treatment of Na(v)1.7-mediated pain in inherited erythromelalgia using a novel sodium channel blocker. Pain 153(1): 80-85.
- Nardi A, Damann N, Hertrampf T, Kless A (2012) Advances in targeting voltage-gated sodium channels with small molecules. ChemMedChem 7(10): 1712-1740.
- Binshtok AM, Bean BP, Woolf CJ (2007) Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449(7162): 607-610.
- Kim HY, Kim K, Li HY, Chung G, Park CK, et al. (2013) Selectively targeting pain in the trigeminal system. Pain 150(1): 29-40
- Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, et al. (2013) Activity-dependent silencing reveals functionally distinct itch- generating sensory neurons. Nature neuroscience 16(7): 910-918.
- Talbot S, Abdulnour RE, Burkett PR, Lee S, Cronin SJ, et al. (2015) Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron 87(2): 341-354.
- Harmer AR, Valentin JP, Pollard CE (2011) On the relationship between block of the cardiac Na(+ ) channel and drug-induced prolongation of the QRS complex. British journal of pharmacology 164(2): 260-273.






























