The Impact of Extraction Method on Antioxidant Activity of Three Herbs in Four Oxidative Systems
NargesJafari Dinai1*, Gholamali Naderi1 and Najme soltanmohammadi2
1Isfahan Cardiovascular Research Center, Isfahan University of Medical Sciences, Iran
2University of Cologne, Cologne Area, Germany
Submission: October 17, 2017; Published: October 26, 2017
*Corresponding author: Narges Jafari Dinai, Isfahan Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran.
How to cite this article: Narges J, Gholamali N, Najme. The Impact of Extraction Method on Antioxidant Activity of Three Herbs in Four Oxidative Systems. J of Pharmacol & Clin Res. 2017; 4(1): 555628. DOI: 10.19080/JPCR.2017.04.555628.
Abstract
Humuluslupulus, CynaraScolymus, Kelussiaodoratissima are traditionally employed to cure various disorders such as oxidative stress. The crude extracts of these three herbs were tested for their potential antioxidant activity and have shown effective antioxidant activity. The main objective of this study is to determine to what extent methods of extraction is effective in antioxidant activity. Given that it is prevalent evaluating the antioxidant activity by using several methods, we used four common methods (DPPH, b-carotene bleaching, NO and OH scavenger) to analyze the antioxidant activity of these extracts that had prepared with two method of extraction (soak and soxhelet methods). Before assaying antioxidant activity, polyphone and flavonoid content of extracts were determined. The results show, in beta-carotene system there isn’t any difference between two methods. In three other systems soxhelet method is better than soak method. According to these results and contents of polyphenol and flavonoid can be concluded, polyphenolic other than flavonoids compounds play role in this antioxidant activity.
Keywords: Oxidative Stress; Antioxidant Activity; Herb
Introduction
Oxidation can be a main issue in foods and a major factor in shortening shelf life by generating off-flavors in lipid containing foods. Oxidation can also lead to loss of color through oxidative degradation of pigments, as well as loss of nutrients and vitamins. It can even change the texture and the functionality by impacting protein structure Lushchak [1]. Using antioxidants constitute a major oxidation defense strategy. Antioxidants compounds blench or inhibit the oxidation of molecules by inhibiting the initiation or propagation of oxidizing chain reactions. The era between the 1920s and 1950s witnessed the start of using antioxidants as individual food additives Gulcin [2]. Generally, there are two basic categories of antioxidants, natural and synthetic. Tendency toward the use of natural additives because of the point of view of safety grows interest to application natural antioxidants Nogochiand Nikki [3]. Herbs have been used for a large range of purposes including medicine, nutrition, flavorings, beverages, dyeing, repellents, fragrances, cosmetics, charms, smoking, and industrial uses and they are one of the most important objects to search for natural antioxidants. It is necessary understanding the chemical nature of the antioxidative defense on the molecular level Naik et al. [4]. Because there is not any simple universal method to measure antioxidant activity exactly and quantitatively Prior et al. [5]. It is prevalent that we evaluate the antioxidant activity of plants by using several methods Schlesier et al. [6]. There have been several studies in the literature reporting antioxidant activity of Humuluslupulus, CynaraScolymus, Kelussiaodoratissima, Jamuna et al. [7] Wittemer et al. [8] Huige et al. [9] Ahmadi et al. [10] Schutz et al. [11] but often in one method. In this experiment, we used four common methods (DPPH, b-carotene bleaching, NO and OH scavenger) to analyze the antioxidant activity of these extracts that prepared with two method of extraction. The main aim of this investigation is to assess antioxidant activities of all three herbs extracts that prepared by two methods in four oxidation system and finally determine that the extraction method to what rate is effective in separating best component with best antioxidant activity.
Material and Method
Herbs were collected and authenticated at the Biology Department, School of Science and Isfahan University. The voucher specimens were deposited in Isfahan University Herbarium.Required parts of the plant were dried at room temperature. The dried plants were ground by an electric blender. In soak method, 100 g of each line of plant powders were soak in 96% and 70% ethanol for 72 h and 12 h respectively. In soxhlet method powders 100g of each plant powder were extracted with a Soxhlet extractor using ethanol 70% for 5 h. After that the extracts were filtered and concentrated by a distiller in a vacuum at 40°C. The resulting solution was vaporized and desiccated in 50°C under sterile conditions. Extract obtained kept in a dark glass bottle at 4°C until use Zhang et al. [12].
Quantitative Phytochemical Analysis
Determination of Total Phenolic Contents
Total phenolic contents in extracts were determined using Folin-Ciocalteau reagent with respect to gallic acid calibration curve. Briefly, 0.2 ml of extract solution and 0.2 ml of Folin-Ciocalteau reagent were added in a test tube and mixed thoroughly. After 4 min, 1 ml of 15% Na2CO3 was added, and then the mixture was allowed to stand for 90 min at room temperature. The absorbance was read at 750 nm and by using an equation obtained from the gallic acid calibration curve, the concentration of the total phenolics was determined Setayesh et al. [13].
Determination of Flavonoid Contents
Total flavonoid contents were measured with the aluminum chloride colorimetric assay 15. At first Quercet in different concentrations (50, 100, 200, 300 and 400 μg/ml) was used for obtaining calibration curve. Each extract was added to 10ml volumetric flask containing4ml of water and 0.3ml of 5% NaNO2 was added. 0.3ml of 10% AlCl3 and 2ml of 1 M NaOH were added After 5 and 6 min respectively and then with distill water total volume was made up to 10ml. After mixing absorbance was measured against a freshly prepared reagent blank at 510 nm. Total flavonoid content of the extracts was expressed as mg of Quercet in equivalent per 100 g dry weight of extract Marinova et al. [14].
Determination of Antioxidant activity
Scavenging activity on DPPH.
Extracts were mixed with 1 ml of DPPH (0/2 mM in ethanol). After shaking to monitor the decrease in absorbance at 517 nm the mixture was placed in anshimadzu-UV 3100 spectrophotometer. Ascorbic acid (Sigma-Aldrich), a stable antioxidant, was used as a synthetic reference. The total stoichiometry of the reaction was calculated employing the formula:
n = A0 – Afε c0l (2)
Where c0 is the initial concentration of phenolic compound (M); A0 the absorbance at 515 nm of the radical at t=0; Af the absorbance at 515 nm of the radical at steady state; ε the molar extinction coefficient for DPPH radical (M-1 cm-1) and l is the optical path of cuvette (cm) Wang et al. [15].
2 B-Carotene bleaching test
The emulsion containing carotene-chloroform, linoleic acid and Tween 40 was added to a tube containing extract and the tubes were placed in a water bath at 50 _C and the oxidation of the emulsion was monitored spectrophotometrically by measuring absorbance at 470 nm over a 90 min period. The antioxidant activity was expressed as inhibition percentage with reference to the control after a 60 min incubation using the following equation:
AA = 100*(DRC _ DRS ) / DRC
AA = antioxidant activity;
DRC = degradation rate of the control = [ln(a/b)/90];
DRS = degradation rate in the presence of the sample = [ln(a/b)/60],
Where a = absorbance at zero time; b = absorbance at 90 min] Oke et al. [16].
Hydroxylradical (.OH)-Scavanging Assay
The hydroxyl radical scavenging activity was assayed according to the method of Lopes, Schulman, and Hermes-Lima. Briefly, each extract was mixed with a solution containing 5 mM 2-deoxyribose, 100 mM H2O2, and 20 mM PBS (pH 7.2). Then, reaction was started by the addition of Fe2+ (6 μM final concentration) to this mixture. The reaction was carried out for 15 min at room temperature and stopped by adding 4% phosphoric acid (v/v) and 1% thiobarbituric acid (TBA, w/v, in 50 mMNaOH). After boiling for 20 min at 95°C, sample was cooled to room temperature and the absorbance was read at 532 nm wang et al. [15].
Nitric oxide radical (NO.) Scavanging Assay
The method of Green 1982 was used to assay the scavenging activity of extracts on nitric oxide. The reaction solution (1 ml) containing 10 mM sodium nitroprusside in PBS (pH7.0) was mixed with extracts at different concentration and followed by incubation at 370C for 1 h. A 0.5 ml aliquot was then mixed with 0.5 ml Griess reagent (1% sulfanilamide in 2.5% phosphoric acid and 0.1% naphthylethylenediaminedihydrochloride). The absorbance at 540 nm was measured. Percent inhibition of nitric oxide generated was measured by comparing with the absorbance value of negative control Lusarczyk et al. [17].
(10 mM sodium nitroprusside and PBS ).100× [Acontrol / (A control – Asample = ( %)]
Results and Discussion
Quantitative results of the extracted substances from the analysed herbs are summarized in (Table 1). The yields of extracts differ from 4.7±0.84 to 15.83±0.56 (gr/100gr dry weight) for Humuluslupulus to respectively that both of them prepared with soxhelet method. In Kelussiaodoratissima, yield has increased in soak method as compared soxhelet method (p= 0.014). Change between two methods doesn’t affect amount of yield in two other herbs. The polyphenol content has increased significantly in CynaraScolymus that prepared with soxhelet method as compared soak method (p=0.03). In two other herbs, kind of extraction doesn’t change amount of polyphenol content.
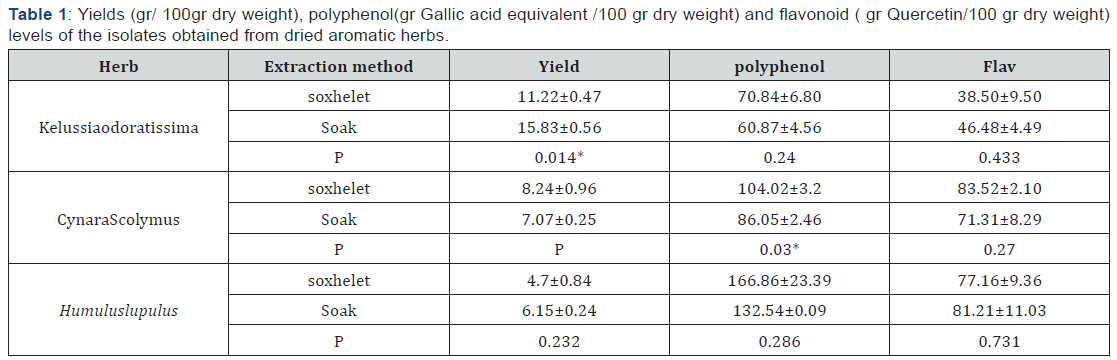
Data are mean ± SD (n = 10).
* : Shows significantly differences between two extraction methods (P < 0.05).
About content of flavonoid in all three herbs, we can say that the kind of extraction doesn’t significantly change amount of flavonoid contents (p≥0.05). In general it can be said that extraction method effect yield and polyphenol contents, and this effect depend on kind of herb. For one herb, soxhelet method and for another herb, soak method is more effective. For some herbs as Humuluslupulus, there isn’t any difference between soak and soxhelet method. The results show that for achieving more amounts of yield, polyphenols and flavonoids contents, choice between extractions methods depend on kind of herb. In CynaraScolymus, polyphenol content has increased in soxhelet method and these results reveal that soxhelet method is more effective in separating polyphenol components.
In this study, antioxidant tests carried out by four test systems, namely, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical scavenging, β-carotene/linoleic acid, No and OH scavenging systems. Hydrogen donating capacity of an antioxidant component was determined in DPPH system that it is best and widely used method for testing free-radical scavenging activity. Donation of hydrogen by an antioxidant cause to form a stable DPPH from radical form of DPPH and this leads to a color change from purple to yellow and a decrease in absorbance Wang et al. [18].
As illustrated in (Table 2), in system DPPH, scavenging activity of extract prepared with soxhelet method (SOX) in CynaraScolymus and Humuluslupulus is more effective than extract prepared with soak method (SOA). In two herbs the more Polyphenol content is seen in soxhelet method compared to soak method, but there is not such difference about flavonid content. Therefore, eventually polyphenol component except flavonoid role-playing in this characteristic. In the other hand, in Kelussiaodoratissima SOA is more effective than SOX. Due to the significant yield of extract in two methods and no significant difference in the amount of flavonoids and polyphenols, we can say, type of isolated flavonoid has been in two different methods or component Apart from polyphenols are effective in creating antioxidant activity.
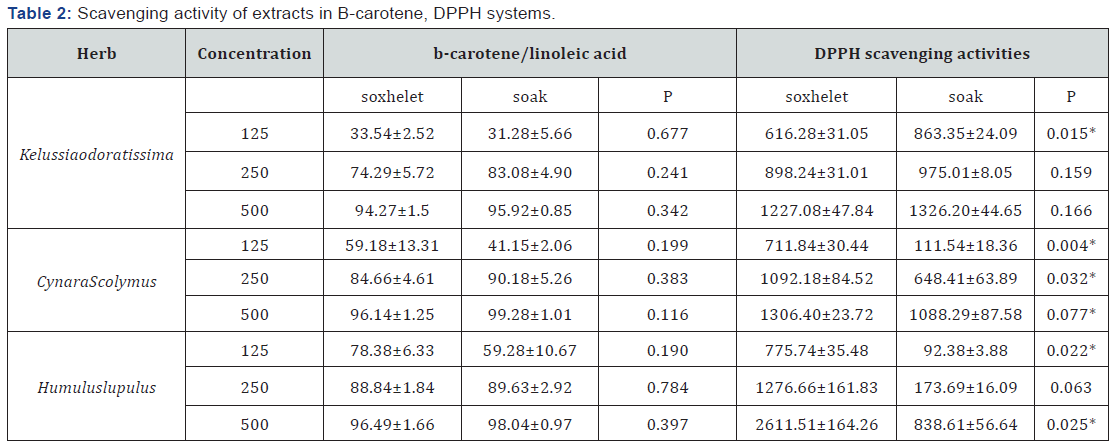
Data are mean ± SD (n = 10).
* : Shows significantly differences between two extraction methods (P < 0.05).
In β-carotene system, in the absence of an antioxidant, β- carotene undergoes rapid discoloration because of attacking linoleic acid radical to unsaturated β-carotene. Oxidation and disruption of β- carotene cause losing orange color which monitored spectrophotometrically. Therefore in samples without antioxidant component, the absorbance decreased rapidly and in the presence of an antioxidant the color was retained for a long time Amiri [19]. In b-carotene/linoleic acid system there isn’t any significant difference between soxhelet and SOA and these results are shown in (Table 2).
Hydroxyl radical is the most representative free radicals and the damaging action of this radical is strongest among free radicals. Hydroxyl radical is usually more susceptible to react with various compounds and actively participate in the initiation of lipid peroxidation Liu and Ng [20]. In scavenging OH systems, in three herbs SOX is more effective than SOA .However, these differences only in Kelussiaodoratissima are significant (p< 0.05). Nitric oxide (NO) is a free radical that can be synthesized from arginine by nitric oxide synthase (NOS). Also, NO and reactive nitrogen species (RNS), derived from the interaction of NO with oxygen or reactive oxygen species, have both been reported to participate in the development of oxidative tissue/cellular damage, which has been established as a mechanism of tissue damage. NO has therefore attracted considerable interests in the field of human health Tsai et al. [21]. In scavenging NO systems, depending on the plant, shift between two different methods affect scavenging activity. For example in Kelussiaodoratissima SOX is more effective than SOA and in Humuluslupulus only in high concentration, SOX is more effective than SOA. In Kelussiaodoratissima and CynaraScolymus at low concentration SOA and at high concentration SOX are better and in Humuluslupulus at low concentration there is not any differences between SOX and SOA, but at high concentrations SOX is more efficiently (Table 3).
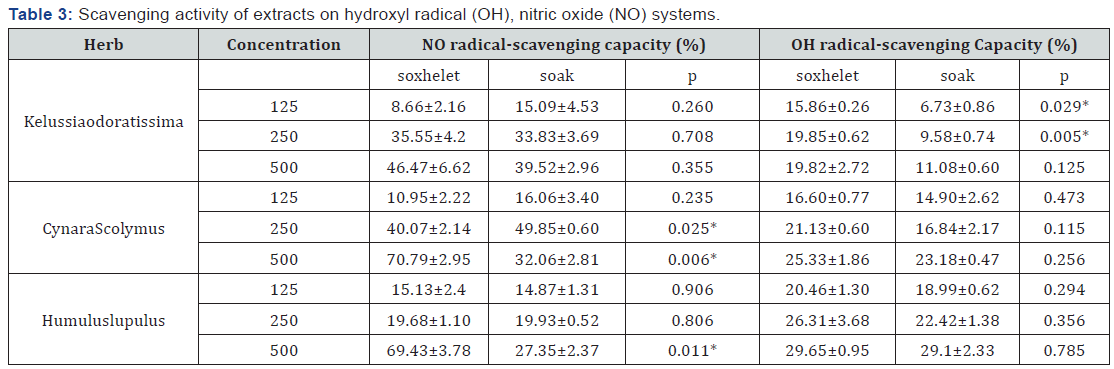
Data are mean ± SD (n = 10).
*: Shows significantly differences between two extraction methods (P < 0.05).
In scavenging OH systems, depending on the plant, shift between two different methods affect scavenging activity. For example in Kelussiaodoratissima SOX is more effective than SOA and in Humuluslupulus only in high concentration, SOX is more effective than SOA. As a general conclusion it can be said that in beta-carotene system there isn’t any difference between two methods. In three other systems antioxidant activity in SOX is better than SOA and according to the content of polyphenol and flavonoid we can say polyphenolic compounds other than flavonoids play a role in this antioxidant activity [22].
References
- Lushchak V (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chemico-Biological Interactions 224: 164- 175.
- Ilhami Gulcin (2010) Antioxidant properties of resveratrol: A structureactivity insight. Innovative Food Science and Emerging Technologies 11(1): 210-218.
- Noguchi C, Nikki E (2000) Phenolic antioxidants: a rationale for design and evaluation of novel antioxidant drugs for atherosclerosis. Free Radical Biology and Medicine 28(10): 1538-1546.
- Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, et al. (2003) Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 63(1): 97- 104.
- Prior RL, Wu XL, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry 53(10): 4290-4302.
- Schlesier K, Harwat M, Bohm V, Bitsch R (2002) Assessment of antioxidant activity by using different in vitro methods. Free Radical Research 36(2): 177-187.
- Jamuna KS, Ramesh CK, Srinivasa TR Raghu KL (2011) In vitro antioxidant studies in some common fruits. International Journal of Pharmacy and Pharmaceutical Science 3(1): 60-63.
- Wittemer SM, Ploch M, Windeck T, Muller SC, Drewelow B, et al. (2005) Bioavilability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine 12(1-2): 28-38.
- Huige Li, Ning Xia, Isolde Brausch, Ying Yao, Ulrich Forstermann (2004) Flavonoids from Artichoke (CynaraScolymusL.) Up-Regulate Endothelial-Type Nitric-Oxide Synthase Gene Expression in Human Endothelial Cells. Journal of Pharmacology and Experimental Therapeutics 310(3): 926-932.
- Fatemeh Ahmadi, Mahdi Kadivar, Mohammad Shahedi (2007) Antioxidant activity of Kelussia odoratissima Mozaff. in model and food systems. Food Chemistry 105(2007): 57-64.
- Schutz K, Kammerer D, Carle R, Schieber A (2004) Identification and quantification of coffeoylquinic acids and flavonoids from artichoke (CynaraScolymus L.) Heads, Juice, and pomace by HPLC-DAD-ESI/MS (n). Agricultural and Food Chemistry 52(13): 4090-4096.
- Zhang Z, Liao L, Moore J, Wu Tao, Wang Z (2009) Antioxidant phenolic compounds from walnut kernels (Juglansregia L.). Food Chemistry 113(2009): 160-165.
- Milad Setayesh, Amir Siahpoosh, Hamid Mashayekhi (2013) Antioxidant activity, total phenolic and flavonoids contents of three herbs used as condiments and additives in pickles products. Herba Polonica 59(3): 1-12.
- Marinova D, Ribarova F, Atanassova M (2005) Totalphenolics and total flavonoids in Bulgarian fruits and vegetables. Journal of the University of Chemical Technology and Metallurgy 40(3): 255-260.
- Wang Z, Hsu C and Yin M (2009) Antioxidative characteristics of aqueous and ethanol extracts of glossy privet fruit. Food Chemistry 112: 914-918.
- Oke F, Aslim B, Ozturk S, Altundag S (2009) Essential oil composition, antimicrobial and antioxidant activities of SaturejacuneifoliaTen. Food Chemistry 112: 874-879.
- Sylwester Lusarczyk, Michal Hajnos, Krystyna Skalicka-Wozniak, Adam Matkowski (2009) Antioxidant activity of polyphenols from Lycopus lucidus Turcz. Food Chemistry 113(2009): 134-138.
- Rufeng Wang, Yi Ding, Ruining Liu, Lan Xiang, Lijun Du (2010) Pomegranate: Constituents, Bioactivities and Pharmacokinetics. Fruit, Vegetable and Cereal Science and Biotechnology 4(2): 77-87.
- Hamzeh Amiri (2012) Essential oils composition and antioxidant properties of three thymus species. Evidence - Based Complementary and Alternative Medicine 224(2012): 164-175.
- Liu F, Ng TB (2000) Antioxidative and free radical scavenging activites of selected medicinal herbs. Life Sci 66(8): 725-735.
- Po-Jung Tsai, Tzung-Hsun Tsai, Chun-Hsien Yu, Su-Chen Ho (2007) Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea. Food Chemistry 103(2007): 181-187.
- Van Der Geer J, Hanraads JAJ, Lupton RA (2000) The art of writing a scientific article. J Sci Commun 163: 51-59.






























