A Comparative Study on Staining Techniques for Vaginal Exfoliative Cytology of Rat
MR Srinivasan*, A Sabarinathan1, A Geetha1, K Shalini2 and M Sowmiya2
1Department of Veterinary Pharmacology and Toxicology, Madras Veterinary College, India
2Department of Pharmacology &Pharmacognosy, Madras Medical College, India
Submission: July 17, 2017; Published: July 27, 2017
*Correspondence Address: MR Srinivasan, M.V.Sc., Assistant Professor, Department of Veterinary Pharmacology and Toxicology, Madras Veterinary College, Chennai - 600 007, Tamil Nadu, India, Tel: +91-7401043980; Email: srinivasan.m.r@tanuvas.ac.in
How to cite this article: M R Srinivasan, A Sabarinathan, A Geetha, K Shalini, M Sowmiya. A Comparative Study on Staining Techniques for Vaginal Exfoliative 004 Cytology of Rat. J of Pharmacol & Clin Res. 2017; 3(3): 555615. DOI: 10.19080/JPCR.2017.03.555615.
Abstract
This study describes the suitable vaginal exfoliative cytology staining technique for the study of various stage of estrus cycle. Two staining techniques namely Crystal violet and Papanicolaou stains were compared and evaluated for various types of cells during the four stage of estrous cycle in rats. The study revealed that the Papanicolaou staining is a better method over crystal violet staining technique, because the Papanicolaou staining is polychromatic, hence it yields clear nuclear and cytoplasmic details of vaginal exfoliativecells. This study concludes that the Papanicolaou staining technique can be utilized for vaginal exfoliative cells staining for the determination various cell stages in estrous cycle in rats.
Introduction
Female rodents are polyestrous, spontaneous ovulators with regular and successive estrous cycles that may vary with age and species. The estrous cycles are also influenced by light, seasons of the year and other environmental factors. Similar to other regularly cycling animals, the changes in ovaries, uterus and the vagina during different phases of estrus cycle [1] occur in rats too within the short cycle length of 4-5 days. The short length of the estrous cycle of rats makes them ideal for investigation of changes occurring during the reproductive cycle [2].The orchestra of various hormones such as estradiol, progesterone, luteinizing hormone and follicle stimulating hormone which is a reflection of the hypothalamic-pituitary-ovarian axis functional status influence the cyclicity of the estrous cycle. Any changes in these hormones normal wave pattern will reflect on the vaginal cell pattern which can be easily identified by observing the daily vaginal exfoliative cytology. Among the various methods by which reproductive status of female rats can be assessed, vaginal cytology is known to be a non-invasive, simple and inexpensive technique utilized for the determination of estrus cycle stages. Evaluation of vaginal cytology under microscope has long been used to record the stages of estrous cycle in the laboratory animals.
Estrus cycle of rats characterized by proestrus, estrus, metestrus (or diestrus I) and diestrus (or diestrus II) [3,4].The cyclic differences in vaginal cytology occur in response to the morphological changes of the vaginal epithelium by cell desquamation.The vaginal mucosal layers consist of stratum corneum, stratum granulosum, rete mucosum and stratum germinativum [3].The proestrus characterized by round nucleated cells of uniform size during this stage vaginal epithelium is consisting of 9 to 12 layers of cells with mature cells at the surface. By the end of protesters the surface layer of mature epithelial cells shed and the stratum corneum is exposed. The estrus lasts for 25 to 27 h and characterized by the presence of irregularly shaped, un-nucleated cornified cells. During the metestrus leukocytes infiltrate the thinned vaginal epithelium owing to a decline in estrogen secretion and pass into the vaginal canal [5] lasting for 6 to 8 h, during this stage vaginal secretion appear white and opaque. Diestrus stage lasts for 55 to 57 h, consists of leucocytes and nucleated cells and the vaginal epithelium reaches its thinnest point (4 to 7 layers). Identification of these stages of estrous cycle in rodents plays an important role in studying the effect of drugs on estrous cycle.
Though there are various methods available for staining the vaginal exfoliative cells, the best method to follow is not clear from the previous literatures. Hence the present study is conducted to compare the two most widely used staining methods such as crystal violet and Papanicolaou staining methodsto differentiate the various stages of oestrous cycle and to determine the better staining method during the studies related to reproductive system in rats.
Materials and Methods
Wistar rats, females (7-9 weeks old) were obtained from Department of Laboratory Animal Medicine, TANUVAS, Madhavaram Milk Colony, Chennai 600 051, Tamil Nadu, India. This experiment was approved by the Institutional Animal Ethics Committee (0520/DFBS/B2015/dated 03-03-2015). All the rats were housed in the air-conditioned room temperature of 23-280 C with 12 h light/12 h dark cycle. Free access to a standard pelleted rat feed and water was provided ad libitum. Maximum of 3 rats per cage was housed in standard shoe-box type cages of laboratory rat. A total of six rats were used for this study. To determine the stages of the estrous cycle, vaginal cytology samples were collected every day at the same time from all the animals for 14 consecutive days as per the procedure by Cooper and Goldman 1999 [6].
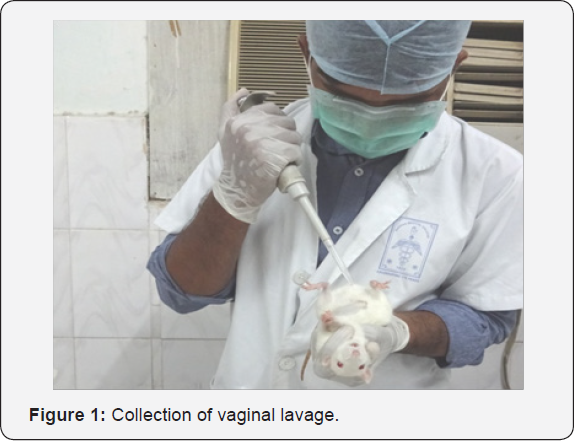
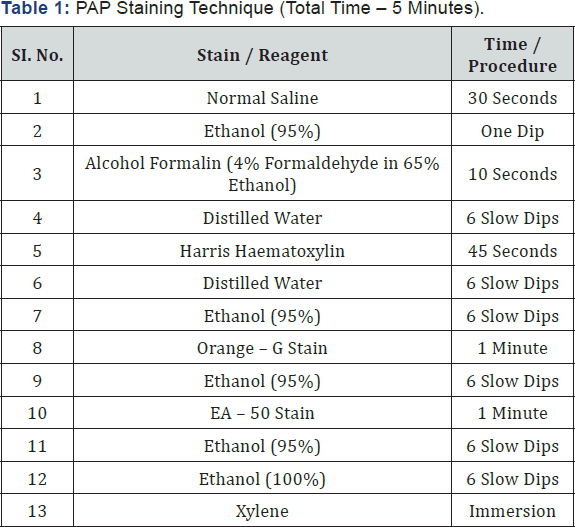
The procedure is as follows, 300 μl of Normal saline was drawn into the disposable micro tips with the help of micropipette. The microtip was gently inserted into vaginal passage at a depth of approximately 5-10 mm and the vaginal passage was flushed 2 or 3 times (Figure 1) with at most care to avoid deep insertion of micro tip which may cause cervical stimulation resulting in induction of pseudo-pregnancy. Following the vaginal lavage samples from each animals were placed evenly on two different microscopic glass slide (Figure2) , air dried for 3-4 hours followed by staining with 0.1% Crystal violet prepared by diluting 0.1 g of Crystal violet in 100 ml of double distilled water followed by filtration through what man filter paper No.1 and stored in tightly sealed container at room temperature as described by [7]or Papanicolaou stain as per the procedure mentioned in the (Table 1) for estrous cycle determination. After staining with both the methods, the slides were observed under light microscope for analysis of the stages of estrous cycle.

Results
Predominant cell type observed in this study during different stage of estrous cycle viz, proestrus with nucleated epithelial cells, estrus cornified squamous epithelial cells, metestrus shows equal proportion of leukocyte, cornified cells and the diestrus smear with predominance of leukocyte as described by [3,8]. Vaginal smears were stained using the Papanicolaou and Crystal violet method are shown in all four stages in (Figure3) . In proestrus, smears stained with Papanicolaou shows nucleated cells with pink cytoplasms, densely packed nucleated cells with dark blue to purple staining granulated nuclei (Figure 3a). Whereas crystal violet showed lightly stained violet colour cytoplasm and darkly pigmented nucleus (Figure 3A). In estrus, cornified squamous epithelial cells were arranged in sheets and clumps, and were uniformly stained pale orange and pink without nucleus when stained with Papanicolaou (Figure 3b). Whereas crystal violet showed uniformly stained cytoplasm appears violet colour without nucleus (Figure 3B).
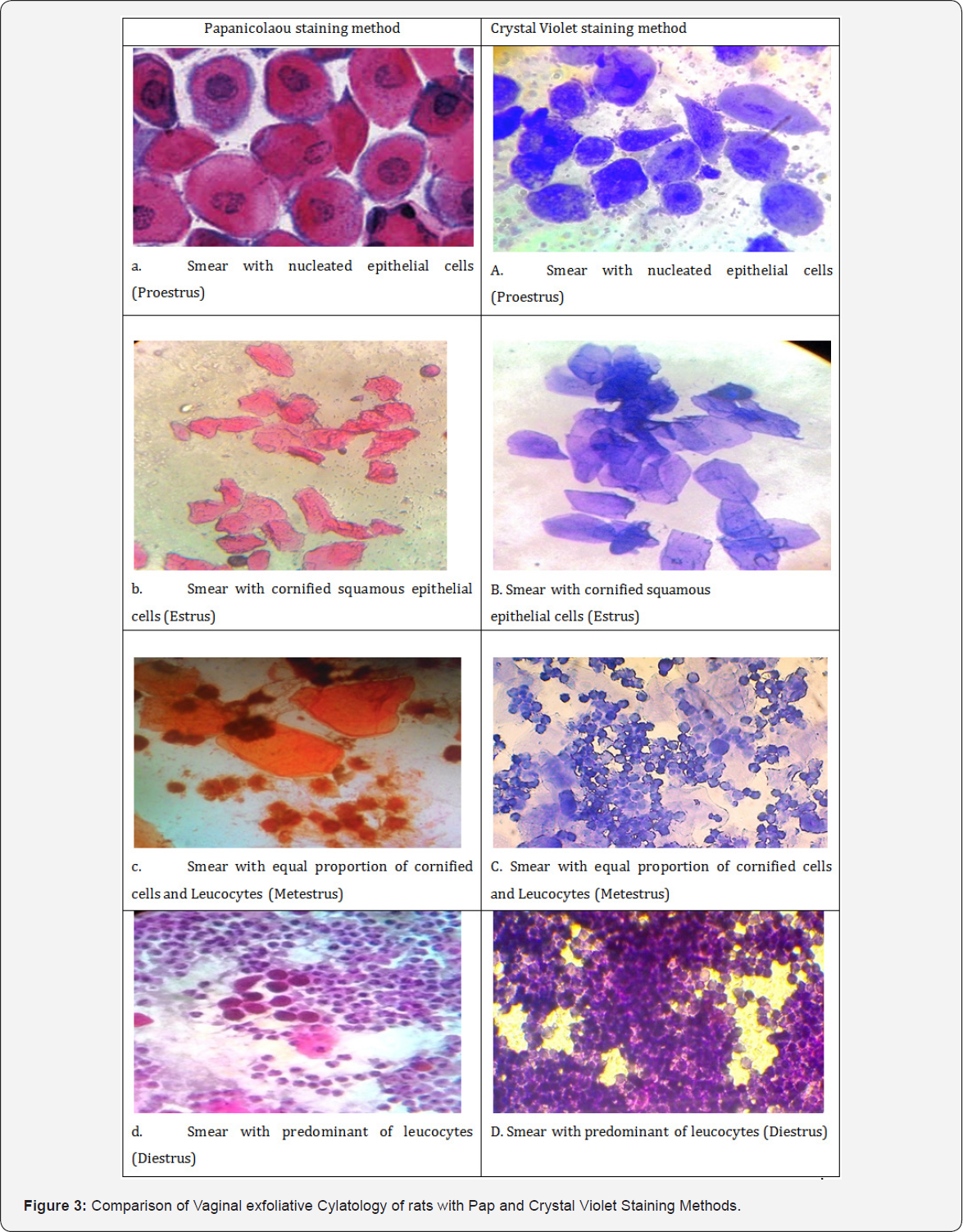
In metestrus, the vaginal exfoliative cytologyrevealed equal proportion of cornified squamous epithelial cells stained orange colour and ruptured leukocytes with darkly pigmented polymorpho nucleus. The nucleated cells in metestrus had a distinct pale blue border surrounding the cell membranes and dark blue stained polymorpho nuclei when smears stained with Papanicolaou (Figure 3c), whereas crystal violet showedviolet colourcornified squamous epithelial cells and leucocytes at equal proportion (Figure 3C).In diestrus, the leukocytes were densely packed in clusters with few nucleated cells, and their abundance gave the smear an overall pink to blue tint when smears stained with Papanicolaou. Leukocytes, as in the metestrus stage, stained dark blue to blue-purple (Figure 3d). Whereas crystal violet showed violet colour densely packed leucocytes with dark blue polymorpho nucleus (Figure 3D). The difference between metestrus and diestrus is the presence of nucleated epithelial cells in diestrus stage.
Discussion
The changes observed in the vaginal cytology are the indication of endocrine events. The cell type and the proportion of cells define the stage of estrous cycle in rat. In the proestrus stage, when mature epithelial cells are shed and predominate in the vaginal smear, during this stage overall pink cast is due to eosin, which stains the cytoplasms of mature squamous cells pink [9]. In estrus, the large cornified cells appear orange due to keratin, which is synthesized during cell differentiation [10]. In metestrusclumping of leukocytes around the epithelial cells is likely due to the action of chemokines, specialized molecules that bind leukocytes in tissues [11]. Chemokines are expressed as a result of luteal regression and are associated with accumulation of leukocytes [11]. In diestrus, the overall appearance of the smear is blue to violet [12]. The study shows that Papanicolaou staining method is superior over the crystal violet staining method in terms of colour changes during different stage of estrous cycle based on age of cell and clear description of the cytoplasmic and nuclear details of the cell can be visualized in the smear stained with Papanicolaou as it is an polychromatic stain which impart colour to both nucleus and cytoplasmic details. The Papanicolaou stain which aids in preserving the smears, and cells are easily described due to their consistent staining pattern. The Papanicolaou stain has been used as a tool to assess vaginal cornification [13].Though swab method of vaginal cytology is more commonly utilized in many studies, in this study lavage method was followed in order to get higher cellularity and also to avoid cervical canal stimulation which may leads to pseudo pregnancy.
Vaginal lavage can be performed not more than once per day as compared to repeated penetration of vaginal canal, aspiration and agitation can cause vaginal irritation, inflammatory response [14] followed by increased levels of leukocyte and leads to disturbance in the vaginal cell may cause incorrect cytological assessment. Three main advantage of Papanicolaou (Pap) staining is good definition of nuclear details, cytoplasmic transparency and indication of cellular differentiation of squamous epithelium. Whereas in crystal violet staining method, the nuclear details and cytoplasmic details are not clearly differentiated, moreover this staining imparts no colour changes to the cells of different stages. Hence it is concluded that the Papanicolaou (Pap) staining method enhances the accuracy in determination of various stage of estrous cycle in rats which is further useful in studying the effect of test compounds on reproductive system in rats.
References
- Marcondes FK, FJ Bianchi, AP Tanno (2002) Determination of the estrous cycle phases of rats: some helpful considerations Braz. J Biol 62(4A): 609-614.
- Hebel R, MW Stromberg (1989) Anatomy and Embryology of the Laboratory Rat. Worthsee: Bio Med Verlag pp. 1-271.
- Long JA, HM Evans (1922) The estrous cycle in the rat and its associated phenomena. Memories of University of California 6: 1-148.
- Freeman ME (1988) The ovarian cycle of the rat. In: E Knobil, J Neil (Eds.), Physiology of reproduction. Raven Press Ltd, New York, USA, 1893-1928.
- Montes GS, EH Luque (1988) Effects of ovarian steroids on vaginal smears in the rat. Acta Anat. (Basel) 133(3): 192-199.
- Cooper RL, JM Goldman (1999) Vaginal cytology. In G Daston, C Kimmel (eds.), An Evaluation and Interpretation of Reproductive Endpoints for Human Health Risk Assessment ILSI Press, Washington, DC, pp. 42-56.
- McLean AC, N Valenzuela, S Fai, SA Bennett (2012) Performing Vaginal Lavage, Crystal Violet Staining and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. J Vis Exp 15(67): e4389.
- Mandl AM (1951) The phases of the oestrous cycle in the adult white rat. Journal of Experimental Biology 28: 576-584.
- Keebler CM, TM Somrak (1993) The Manual of Cytotechnology. (7th ed). American Society of Clinical Pathologists Chicago.
- Gimenez-Conti IB, M Lynch, D Roop, S Bhowmik, P Majeski et al. (1994) Expression of keratins in mouse vaginal epithelium. Differentiation 56(3): 143-151.
- Townson DH, AR Liptak (2003) Chemokines in the corpus luteum: implications of leukocyte chemotaxis. Reprod Biol Endocrinol 1: 94.
- Hubscher CH, DL Brooks, JR Johnson (2005) A quantitative method for assessing stages of the rat estrous cycle. Biotechnic and Histochemistry 80(2): 79 to 87.
- Chateau D, JM Geiger, B Samama, N Boehm (1996) Vaginal keratinization during the estrous cycle in rats: a model for evaluating retinoid activity. Skin Pharmacol 9(1): 9-16.
- Yano J, E Lilly, M Barousse, PL Fidel (2010) Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infection and immunity 78(12): 5126-5137.






























