Daclizumab is more effective in Reducing Relapse Rates in Multiple Sclerosis Patients than Interferon Beta-1a
Jerry Silverman*, Joseph Gulfo* and Jigar Amin
Department of Pharmacy, Fairleigh Dickinson University, USA
Submission: April 21, 2017; Published: May 30, 2017
*Corresponding author: 1.Jerry Silverman, Department of Pharmacy, Fairleigh Dickinson University, 230 Park Ave, Florham Park, NJ 07932, USA, Tel: 973-443-8401; Email: jesilver@fdu.edu
2. Joseph Gulfo, Department of Pharmacy, Fairleigh Dickinson University, 230 Park Ave, Florham Park, NJ 07932, USA,Email: jvgulfo@fdu.edu
How to cite this article: Jerry S, Joseph G,Jigar A. Daclizumab is more effective in Reducing Relapse Rates in Multiple Sclerosis Patients than Interferon Beta-1a. J of Pharmacol & Clin Res. 2016; 3(1): 555601. 10.19080/JPCR.2017.03.555601
Abstract
Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) that causes demyelination of neurons, leading to temporary or permanent paralysis. It is believed that T-cells play an important role in deterioration of the myelin sheath around each neuron. Recent findings have suggested that the IL-2 receptor plays an integral role in relapsing remitting MS (RRMS). Daclizumab is used in MS patients that have RRMS. It is an IL-2 receptor modulator that has been shown to reduce relapse rates. Compared to interferon beta-1a, daclizumab is considered to be highly efficacious in MS patients. It has also been shown that daclizumab reduces magnetic resonance imaging activity. However, its use is limited due to risk of autoimmune disorders and hepatotoxicity. Considering its benefits in MS patients, use of daclizumab can outweigh its risks, provided that it is used in the context of a strict monitoring program. Daclizumab is a better option over interferon beta-1a for patients with active MS.
Abbreviations: MS: Multiple Sclerosis; CNS: Central Nervous System; RRMS: Relapsing Remitting MS; SPMS: Secondary Progressive Stage; MRI: Magnetic Resonance Imaging; EDAA: Expanded Disability Status Scale; HYP: High-Yield Process
Introduction
Multiple Sclerosis (MS) is an autoimmune disease in which the immune system attacks the central nervous system (CNS), leading to demyelination of neurons [1]. MS has no distinct pattern of demyelination; rather, it occurs randomly throughout the white matter resulting in a range of symptoms. Following the demyelination, the central nervous system undergoes partial remyelination of neurons [2-5]. However, the attack by immune cells is very severe, ultimately causing neurodegeneration [5]. MS is divided into two stages:
- relapsing remitting stage (RRMS) and
- Secondary progressive stage (SPMS)
Initially, the condition of neurons slowly deteriorates and symptoms often appear during the attack of immune system and slowly resolve follow remyelination [2]. With SPMS, on the other hand, is characterized by a relentless and constant attack on the myelin sheaths [2]. During this stage, the attack is so frequent that it is very challenging for the CNS to recuperate from the myelin loss [2]. At this stage, MS patients often experience many symptoms at a higher rate, which results in demyelination and neuronal loss, leading to temporary or permanent loss of function [2]. Many lines of evidence indicate that T-cells play a major role in the demyelination of neurons, and can cause a significant increase in the size of lesions [2]. Interleukin-2 is one of the first principle cytokines that regulates T-cell proliferation and function. Daclizumab is an IL-2 receptor modulator that prevents T-cell proliferation in MS patients. Without any interference, T-cells further proliferate and divide into subsets, which include T-helper 1 cells (Th1) and Th2 cells [6]. It is suspected that Th1 cells that produce Interferongamma have a crucial role in attacking oligodendrocytes and myelin in active multiple sclerosis [6]. Interferon beta-1a, helps prevent interferon gamma to attacks on oligodendrocytes and myelin sheath in MS patients. Substantial evidence reveals that daclizumab is more effective in reducing relapse rates in MS patients than Interferon beta-1a [7].
It is hypothesized that daclizumab has a higher affinity for interleukin-2 receptor and it inhibits signals that induce T-cell? proliferation and activity to a greater extent than interferon beta-1a in RRMS [7]. Daclizumab provides sustained benefit and less toxic than IL-2 inhibitors that were developed [7]. Moreover, daclizumab demonstrated excellent results as a single agent in studies of patients with RRMS [7]. Interferon beta-1a, on the other hand, can interfere with cell signaling, resulting in, and flare-ups [7]. It is widely used as a first line of agent in treatment for relapsing-remitting MS since it reduce the relapsing stages by 30% and has a better safety profile than interferon beta-1a [7]. Moreover, it is believed that interferon beta-1a reduce the overall accumulation of brain lesions, which is measured through magnetic resonance imaging (MRI) [89]. However, the mechanism of action of interferon beta-1ais not fully understood and need further studies. A phase 3 study comparing interferon beta-1a and daclizumab was conducted in RRMs. Patients has cranial magnetic resonance imaging showing with a score of 0 and 5.0 on Expanded Disability Status Scale (EDAA) [7]. Participant had two or more clinical relapses within 3 years, at least one clinical relapse appearing within 12 months before randomization, one or more clinical relapses and at least one new lesion on MRI that was not associated with the clinical relapse within the previous 2 years, with at least one of these events occurring in the 12 months before randomization [7].
Patients were randomized to receive either daclizumab high-yield process (HYP) at a dose of 150 mg every 4 weeks and intramuscular placebo once weekly or intramuscular interferon beta-1a at a dose of 30 micrograms once weekly and subcutaneous placebo every 4 weeks for at least 96 weeks and no more than 144 weeks [7]. Patients in this study continued to take this treatment for up to 144 weeks [4]. All efficacy assessments were evaluated by trained, certified, and examining neurologists who were not part of the care of the patients in the study of this article [7]. The EDSS score was determined at screening every 12 weeks and during unscheduled visits for suspected relapse [7]. MRIs were obtained at baseline and at weeks 24, 96, and 144 to evaluate brain lesion progression [7]. The primary end point was the annual relapse rate over 144 weeks [7]. The secondary end point in this study was the number of new lesions evaluated from MRI scans over period of 96 weeks [7]. A total of 1841 patients were enrolled in this study. 919 received daclizumab HYP group and 922 received interferon beta-1a group.
Relapse rates were much lower in the daclizumab HYP group than the one in the interferon beta-1a group (0.22 vs. 0.39, representing a 45% lower rate with daclizumab HYP; P<0.001). The number of new lesions at weeks 96 was 54% lower for patients in the daclizumab HYP groups than in the interferon beta-1a group [7]. Additionally, the secondary findings were also significant. The number of new or newly enlarged hyperintense lesions in T2-weighted images at week 96 was 54% lower in the daclizumab HYP group than in the interferon beta-1a group (P<0.001) [7]. More detailed information pertaining to the results is shown in Table 1. Adverse events were similar in the daclizumab HYP group and in the interferon beta-1a group and included nasopharyngitis, headache, upper respiratory tract infection, pyrexia, injection site pain, and influenza-like illness. There was a higher rate of infections in patients taking daclizumab than the interferon beta-1a. Five patients (1%) in the daclizumab had to discontinue treatment due to development of the severe cases of infections. Overall, the primary and secondary endpoints seemed favorable for daclizumab than its active comparator, interferon beta-1a [7]. In other words, the number of relapse rates and number of lesions were significantly lower in the patient group with daclizumab therapy. Despite its favorability with treatment for relapse-remitting MS, daclizumab showed a growing concern for toxicity and infections. Results in the article showed that 65% of the patients on daclizumab showed serious infections as compared to only 57% of the patients on interferon beta-1a.
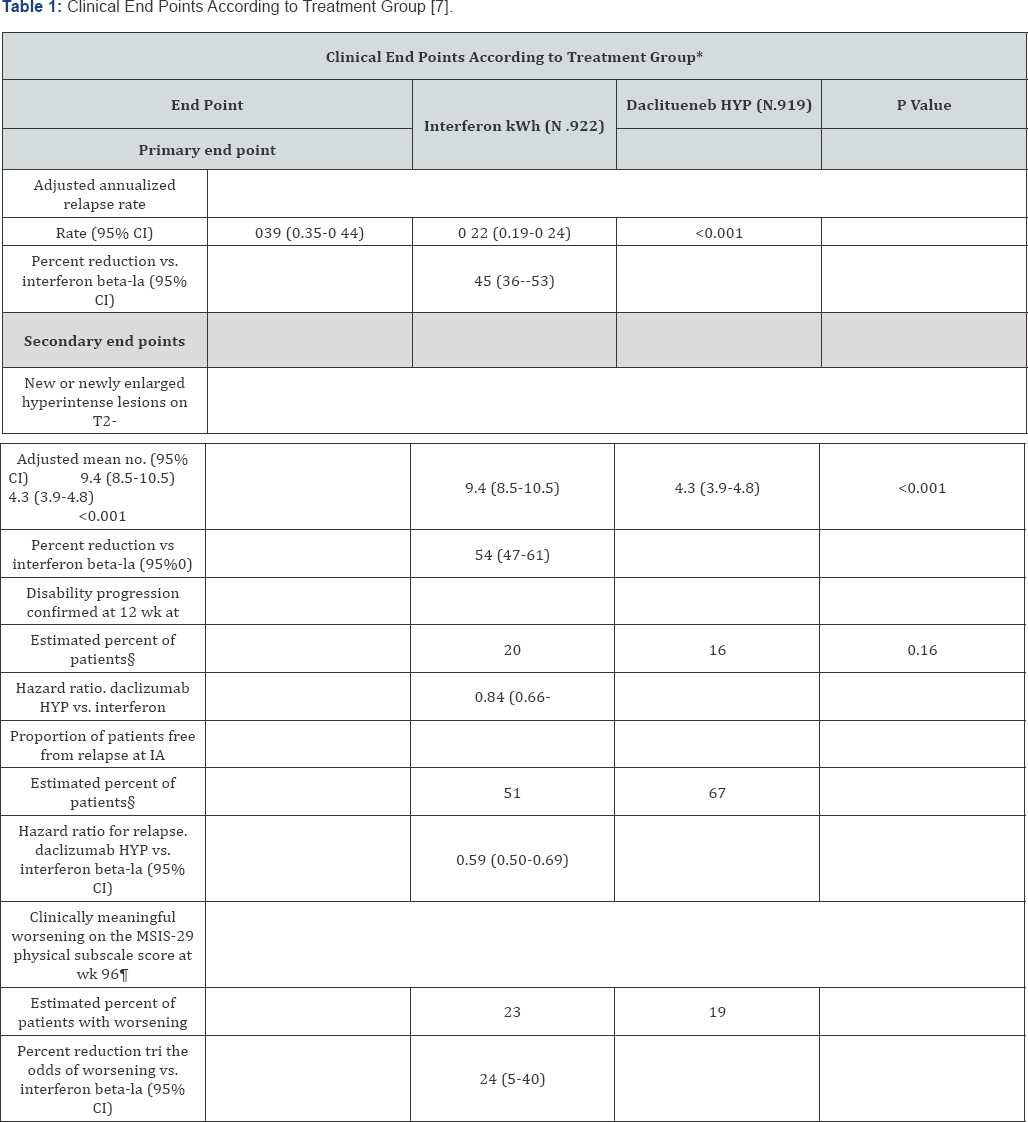
*The number of patients is the intention-to-treat population for each treatment group, excluding patients with missing data for baseline covariates unless otherwise noted. CI denotes confidence interval.
†Secondary end points were rank-ordered (in the order listed here) and were tested with the use of a sequential dosed-testing procedure. If a comparison did not indicate significance (at the 0.05 significance level), all lower-ranked end points were not considered to be statistically significant within the closed-testing procedure. Thus, the end points for the percentage of patients who were relapse free at week 144 and for clinical worsening on the basis of MSIS-29 physi-cal subscale score were not tested because the P value for the secondary end point of disability progression confirmed at 12 weeks was 0.16.
‡The analysis was performed in the subgroup of the Intention to-treat population who had a post baseline scan, which included 841 patients in the interferon beta la group and 864 in the daclizumab HYP group.
§The percentage of patients was estimated by means of the Kaplan-Meier product-limit method.
¶Clinically meaningful worsening was defined as an increase of at least 7.5 points from baseline in the MSIS-29 physical subscale score. Data for 10 patients in the interferon beta-la group and for 13 in the daclizumab HYP group were ex-cluded owing to missing baseline covariates.
Discussion
Daclizumab reduces relapses and the number of MRI lesions in RRMS significantly compared to interferon beta-1a. Despite its superior efficacy profile use of daclizumab was associated with a higher rate of infections. Some of the serious infections include urinary tract infection, pneumonia, appendicitis, cellulitis, and viral infection [7]. Use of monitoring program could mitigate the risks associated with the use of daclizumab so that patients can benefit from its superior effectiveness. This type of toxicity profile for daclizumab allows healthcare providers to implement a monitoring program, which will continue to monitor this drug during treatment while providing patients with best effective medication available for relapse-remitting Multiple Sclerosis.
Acknowledgement
The author would like to thank Dr. Joseph Gulfo and Mr. Jerry Silverman for their excellent skills in reviewing the manuscript.
References
- Stancic M, van Horssen J, Thijssen VL, Gabius HJ, van der Valk P, et al. (2011) Increased expression of distinct galectin in multiple sclerosis lesions. Neuropathology and Applied Neurobiology 37(6): 654-671.
- Compston A, Coles A (2002) Multiple Sclerosis. Lancet 359(9313): 1221-1231.
- Yang L, Anderson DE, Kuchroo J, Hafler DA (2008) Lack of TIM-3 Immunoregulation in Multiple Sclerosis. J Immunol 180(7): 44094414.?
- Chen Zhu, Ana C Anderson, Anna Schubart, Huabao Xiong, Jaime Imitola, et al. (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6(12): 1245-1251.
- Ludwig Kappos, Heinz Wiendl, Krzysztof Selmaj, Douglas L Arnold, Eva Havrdova, et al. (2015) Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 373: 1418-1416.
- Irvine KA, Blakemore WF (2008) Remyelination protects axons from demyelination-associated axon degeneration. Brain 131: 1464-1477.
- Stubbins RE, Holcomb VB, Hong J, Nunez NP (2011) Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 51(7): 861-870.
- Yang RY, Yu L, Graham JL, Hsu DK, Lloyd KC, et al. (2011) Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity. Proc Natl Acad Sci USA 108(46): 18696-18701.
- Wada J, Kanwar YS (1996) Identification and Characterization of Galectin-9, a Novel p-Galactoside-binding Mammalian Lectin. J Biol Chem 272(9): 6078-6086.





























