Drug-Drug Interaction studies of Levocetirizine with Losartan Potassium
Khalid Aftab*1, Shafaque Mehboob2, Afshan Mehboob Khan3, Najma Sultana4 and Syed Arayne4
1Department of Pharmacology & Therapeutics, Islam Medical & Dental College, Sialkot, Pakistan
2Institute of Pharmaceutical Sciences, Jinnah Sindh Medical University, Karachi, Pakistan
3Department of Physiology, Sir Syed Medical College, Karachi, Pakistan
4Department of Pharmaceutical Chemistry, University of Karachi, Karachi, Pakistan
Submission: March 26, 2017; Published: April 12, 2017
*Corresponding author: Khalid Aftab, Professor & HoD Pharmacology & Therapeutics, Islam Medical & Dental College, Sialkot - 51311, Pakistan, Email: khalidaftabkhan@hotmail.com
How to cite this article: Khalid A, Shafaque M, Afshan M K, Najma S , Syed A.Drug-Drug Interaction Studies of Levocetirizine with Losartan Potassium. J of Pharmacol & Clin Res. 2017; 2(4): 555595 . DOI: 10.19080/JPCR.2017.02.555595
Abstract
Objective: The objective of the study was to evaluate the drug-drug interaction studies of Levocetirizine with Losartan potassium
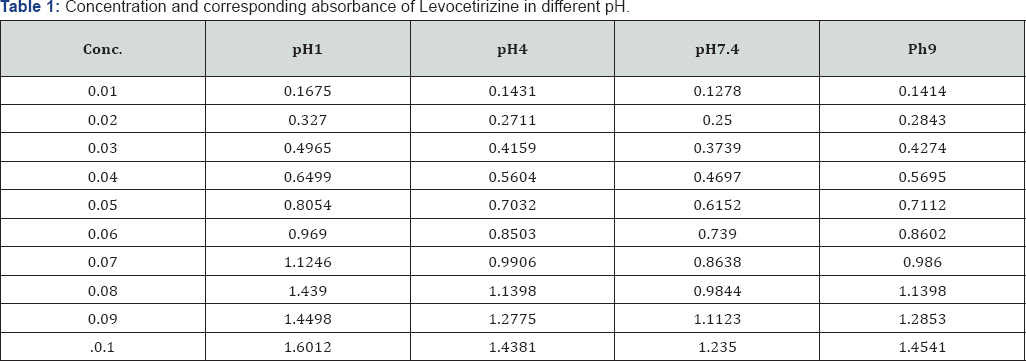
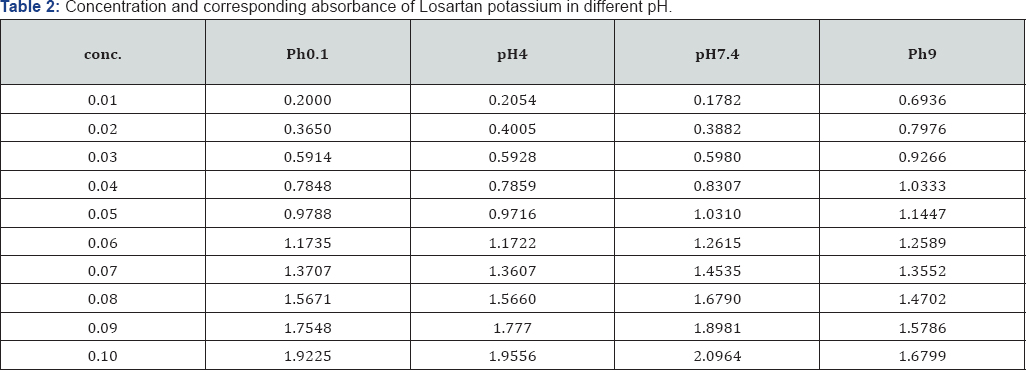
Methodology: Calibration curve studies of working standard solutions of Levocetirizine and Losartan potassium (0.01-0.1 m Mole) were scanned. Maxima appeared at 231nm for Levocetirizine and 205 nm for Losartan potassium. The calibration curve obeyed Beer Lambert's Law. Lone availabilities of both the drugs were studied in pH 1, pH4, and pH 7.4 and pH9 at 37°C on B.P dissolution apparatus. To study the drug- drug interaction of Levocetirizine (5mg tablet) with Losartan potassium (50mg tablet), both the drugs were introduced to the dissolution apparatus at zero time and the absorbance maxima were measured at the corresponding wavelength. Graphs were plotted for % availability of drug versus time at each set of experiment.
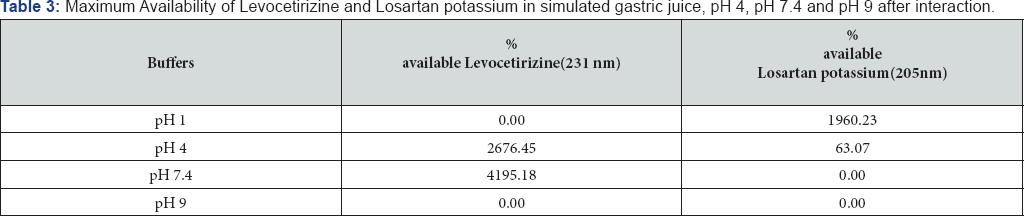
Results: The % availability of Levocetirizine in the buffers of pH simulated to gastric juice, pH 4and pH 7.4 in the presence of Losartan potassium was 0.00%, 2676.45% and 4195.18% respectively but the availability of losartan potassium was increased up to 1960.23% in simulated to gastric pH and decreased upto and 63.07%and o% in the buffers of pH 4, and 1 respectively. Maximum interaction is observed at pH 9 i.e., o%.
Conclusion: On the basis of these studies, it is concluded that Levocetirizine form a charge-complex with Losartan potassium; therefore, co-administration of these drugs should be avoided.
Keywords: Levocetirizine; Losartan potassium; Drug-Drug Interactions; Absorbance Maxima
Introduction
Levocetirizine is a third generation non-sedative antihistamine, developed from the second generation anti-histamine cetirizine, works by blocking histamine receptors. It does not prevent the release of histamine from mast cell, but prevent its binding from its receptors. This in turn prevents the release of other allergic chemicals and increased blood supply to the area, and provides relief from the typical symptoms of hay fever and used to manage intermittent and persistent allergic rhinitis [1]. The qualitative assays of Levocetirizine have been performed in different subjects to determine the anti-allergic activity of the drug [2-7]. Ultraviolet (UV) detection was performed for the quantification of Levocetirizine in the tablets and for enantiomer purity testing of the drug by a validated, selective, precise and accurate method [8].
For the treatment of patients with seasonal and perennial allergic rhinitis with or without concurrent asthma, Levocetirizine was reported 5mg once daily for 32 days. Alleviation and improvement of the symptoms such as rhinorrhea, sneezing, conjunctivitis, and asthmatic symptoms were observed in over 80% of the patients at the end of the experiment [9,10]. As compared to cetirizine, Levocetirizine of 5mg dosage is Pharmacokinetically equals to 10 mg cetirizine [11]. Levocetirizine and Dextrocetirizine may have consequences for drug interactions at the renal level [12]. Levocetirizine is a weak P-glycoprotein substrate; therefore, it should be taken with cautions with the drugs which are either PgP substrate such as Ketoconazole, Cyclosporine or Verapamil or PgP inducers like rifampicin or inhibitor such as Erythromycin, Azithromycin or Itraconazole [13,14].
Losartan potassium is antihypertensive drug belongs to Angiotensin receptor antagonist. It produces its action by blocking angiotensin subtype [1]. Its adverse effects include headache, upper respiratory infections, dizziness, fatigue, and cough [15,16]. Many sensitive methods are reported for the quantitative determination of losartan in different dosages as well as samples including spectroflurometric method, Reverse Phase-High Performance Liquid Chromatography method, APCI method and RP-HPLC method [17,18]. Losartan interact with Ritonavir and Nelfinavir. It has also tendency to interact with H2 receptor antagonists proton pump inhibitor and Atorvastatin [19,20]. The object of present work is to evaluate the possible drug-drug interactions of Levocetirizine with Losartan potassium if used co-administered.
Methodology
To study drug interactions, reported methods were followed [21]. Reference standard of Levocetirizine was gifted by Hilton Pharma (Pvt.), Karachi, whereas, Losartan potassium was given by Zafa Pharmaceutical Laboratories (Pvt.) Ltd, Karachi. Each product was labeled properly and expiry dates checked and they were not earlier than two years old at the time of study. All the reagents used were of analytical grade and all the glassware were used of Pyrex brand.
Equipment: Using the following calibrated equipment's, analytical measurements were carried out. Electrical balance [Mettler Toledo AB54], pH meter [Mettler Toledo MP220], UV visible spectrophotometer [Model 1606, Shimadzu, Japan] with 10 mm path length connected to a P-IV computer loaded with Shimadzu UVPC version 3.9 software was used in these studies, 1 cm rectangular quartz cells, ground glass distillation assembly, water distillation unit [GEL type 2001/2, No. 10793600G], melting point apparatus [Gallenkamp] and deionizer [Stedec CSW-300] were used. The dissolution equipment was manufactured to the B.P 2007 standard.
Preparation of solutions: Levocetirizine 0.04254 gm and Losartan potassium 0.0461gm weighed accurately and each drug was dissolved in one liter of buffers of pH 1-9 to get primary solution of 1 m Mole, from that the stock solution of 0.1 m Mole was prepared by diluting 25 ml of primary solution into 250 ml volumetric flask containing corresponding buffers. Different dilutions ranging from 0.01 to 0.1 m Mole were prepared by diluting the stock solutions (0.1 m Mole) with different buffer solutions of pH 9. For this purpose 5, 10, 15, 20, 25, 30, 35, 40 and 45 ml of stock solutions were separately pipette out in nine different 50 ml volumetric flasks and diluted with individual buffer solutions up to the mark to prepare the working solutions of 0.01-1.0mMole. These solutions were used for calibration curve studies.
Calibration curve studies: working standard solutions of both the drugs of 0.01-0.1 mMole were prepared for this purpose. The absorbance maxima were scanned in the region of 200-700 nm against the reagent blank. Maxima appeared at 231 nm for Levocetirizine and 205 nm for Losartan potassium. The calibration curve was plotted for absorbance against concentration and straight lines were obtained which obeyed Beer Lambert's Law. Epsilon value was also calculated from these observations. In vitro availability studies: the in vitro availability of Levocetirizine was studied in simulated gastric juice (pH 1), pH 4, pH 7.4 and in pH 9 at 37°C on B.P dissolution apparatus. 5mg of Levocetirizine was introduced in 1 liter dissolution medium. Aliquots of 5 ml were withdrawn intermittently at 15 minutes time intervals for 120 minutes and assayed for the drug contents. The volume of the dissolution fluid was maintained by adding an equivalent amount of dissolution fluid withdrawn in the same bath at the same temperature. The sample was scanned in the region 200-700 nm against blank. The same procedure was adopted to calculate the availability of 50mg of Losartan potassium tablet.
Drug-drug interaction studies of Levocetirizine and Losartan potassium: To study the drug-drug interaction of Levocetirizine (5 mg tablet) and Losartan potassium (50 mg tablet), both the drugs were introduced to the dissolution medium at zero time. Same procedure was adopted to measure the absorbance maxima of both the drugs at the corresponding wavelength. (Figures 1& 2) were also plotted for % availability of drug versus time at each set of experiment.


Results
Levocetirizine and Losartan potassium interfere at each other's wavelength. The lone availability of both the drugs in all the pH calculated not more than 115%. After the interaction, availabilities of Levocetirizine as well as Losartan potassium increased in the presence of each other in simulated gastric juice and the rest of the buffers. At the start of experiment in simulated gastric juice, 61.95% of the drug was available which exceeded to 436.78% till the end of the experiment. Similarly, in the buffers of pH 4, 7.4 and 9, an increased availability of Levocetirizine was observed i.e., 376.90%, 436.78% and 436.78% respectively. The availability of Losartan potassium was increased up to 2395.95% , 4195.18% 1960.23 and 61.90% in simulated to gastric pH and in the buffers of pH 4, pH 7.4 and pH 9 respectively (Figure 3)(Tables 1-3).

Discussion
This procedure was design to simultaneously measure the quantities of two drugs present in the same solution without separating them. This was accomplished by developing the mathematical relationship between Levocetirizine and interacting drug because both the drugs have interfered at each other's wavelength which gave the concentration of two drugs simultaneously, when maxima measured at their absorption. Molar absorptivities were used in calculating the quantities of these drugs in a solution of unknown concentration.
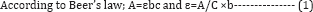
Where, A= absorbance, ε= molar absorptivity or epsilon, b= path length of the cell (1cm) & c= concentration of the solution.
If more than two components are present in the solution which was absorbing at the same wavelength, the above equation (1) can be written as;
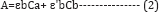
Where, Ca and Cb were concentrations of the two components present in the solution and ε and ε' were the absorptivities of the two components obtained from the absorbance of the standard solution. Similarly, this equation could be derived for the absorbance taken at another wavelength.
Levocetirizine absorbs maximum at 231nm and Losartan Potassiu mat 205 respectively. Let Ca be the concentration of Levocetirizine and Cb is of Losartan potassium. Now equation (2) can be written as;

Where, b is 1, and a1 and a2 were absorptivities of Levocetirizine at 231nm and 205nm and b1 and b2 were of Losartan Potassium at 231nm and 205nm. By multiplying equation (3) with a2 and equation (4) with a1, we get;
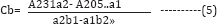

The above equations (5) and (6) were used to calculate % availabilities of Levocetirizine and Losartan potassium in the presence of each other [22]. Both the drugs showed more than 200% availability that is impossible. This may be due to the formation of charge-transfer complex. Therefore, the resultant chelate proved the drug-drug interaction of Levocetirizine with Losartan potassium in different pH. Therefore, precaution should be taken by a hypertensive patient while using Levocetirizine (an anti-allergic drug) with Losartan Potassium. In vivo and large scale studies are highly recommended because as a result of interaction both the drugs can either decrease or even lose their therapeutic effects.
Conclusion
Several studies are reported which indicate that Levocetirizinedi hydrochloride has tendency to form complex with other drugs or substances [22,23]. On the other hand, availability of Losartan Potassium has also been observed to be influenced in the presence of other drugs [24,25]. Keeping this fact in view, current study is conducted which also supports the drug-drug interactions of Levocetirizine and Losartan Potassium. On the basis of these studies, it is concluded that Levocetirizine is a form of charge-complex with Losartan potassium; therefore the co-administration of these drugs should be avoided.
References
- Block J. and Beale JM (2003) Wilson & Gisvold’s Textbook of Organic Medical and Pharmaceutical Chemistry, (11th edn), pp. 10: 675.
- Ciprandi G, Cirillo I, Vizzaccaro A, Civardi E, Barberi S, et al. (2005) Desloratadine and Levocetirizine improve nasal symptoms, airflow, and allergic inflammation in patients with perennial allergic rhinitis: A pilot study. Int Immunopharmacol 5(13-14): 1800-1808.
- Cranswick N, Turzikova J, Fuchs M, Hulhoven R (2005) Levocetirizine in 1-2 year old children: Pharmacokinetic Pharmacodynamic profile. Int J Clin Pharmacol Ther 43(4): 172-177.
- Hulhoven R, Rosillon D, Letiexhe M, Meeus MA, Daoust A, et al. (2007) Levocetirizine does not prolong the QT/QTc interval in healthy subjects. Eur J Clin Pharmacol 63(11): 1101-1107.
- Verster JC, Volkerts ER, Oosterwijck AW, Aarab M, Bijtjes SI, et al. (2003) Acute and Sub-chronic effects of Levocetirizine and diphenhydramine on memory functioning, psychomotor performance and mood. J Allergy ClinImmunol 111(3): 623-627.
- Purohit A, Melac M, Pauli G, Frossard N (2003) Twenty-four-hour activity and consistency of activity of Levocetirizine and Desloratadine in the skin. Br J Clin Pharmacol 56(4): 388-394.
- Ulrich W, Eric B, Roger A, Marie CP (2005) Levocetirizine in children: evidenced efficacy and safety in a 6 week randomized seasonal allergic rhinitis trial. Pediatric Allergy and Immunology 16(3): 267-275.
- Van Eeckhaut A, Michotte Y (2006) Chiral separation of Levocetirizine by capillary electrophoresis. Electrophoresis 27(12): 2376-2385.
- Dhakam Z, McEniery CM, Yasmin, Cockcroft JR, Brown MJ, et al. (2006) Atenolol and Eprosartan: Differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens 19(2): 214-219.
- Devalia J, DeVos C, Hanotte F, Baltes E (2001) A randomized, DoubleBlind, Crossover Comparison among Cetirizine, Levocetirizine, and UCB 28557 on Histamine-Induced Cutaneous Responses in Healthy Adult Volunteers. Allergy 56(1): 50-70.
- Bachert C, Bousquet J, Canonica GW, Durham SR, Klimek L, et al. (2003) Levocetirizine improves quality of life and reduces costs in long-term management of persistent allergic rhinitis. J Allergy Clin Immunol 114(4): 838-844.
- Strolin Benedetti M, Whomsley R, Mathy FX, Jacques P, Espie P, et al. (2007) Stereo selective renal tubular secretion of Levocetirizine and Dextrocetirizine, two enantiomers of the HH1-antihistammiine cetirizine. Fundam Clin Pharmacol 22(1): 19-23.
- Thiessen BQ, Wallace SM, Blackburn JL, Wilson TW (1990) Increased prescribing of antidepressant, subsequent to p-blocker therapy. Arch Intern Med 150(11): 2286-2290.?
- Abdinee H, Sultana MA, Hefnawy MM, Bilal F (2005) Spectroflurometric determination of some p-blockers in tablets and human plasma using 9, 10-dimethoxyanthracene-2-sodium Sulfonate. Die Pharmazie 60(4): 265-268.
- Ohtawa M, Takayama F, Saitoh K, Yoshinaga T, Nakashima M (1993) Pharmacokinetics And Biochemical Efficacy After Single And Multiple Oral Administration Of Losartan, An Oral Active Nonpeptide angiotensin II receptor antagonist in humans. Br J Clin Pharmacol 35(3): 290-297.
- Goldberg MR, Tanaka W, Barchowsky A, Bradstreet TE, McCrea J, et al. (1993) Effects of losartan on blood pressure, plasma renin activity, and angiotensin II in volunteers. Hypertension 21(5): 704-713.
- Furtek CI, Lo MW (1992) Simultaneous determination of a novel angiotensin II receptor blocking agent, losartan, and its metabolite in human plasma and urine by high-performance liquid Chromatography J Chromatogr 573: 295-301.
- Ritter MA, Furtek CI, Lo MW (1997) An improved method for the simultaneous determination of losartan and its major metabolite, EXP3174, in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal 15: 1021-1029.
- Unger T, Kashchina, Elena (2003) Drug interaction with angiotensin receptor blocker; Acomparison with other antihypertensive drugs. Drug Safety 26(10): 707-720.
- Fichtenbaum CJ, Gerber JG (2002) interaction between antiretroviral drugs and drugs used for therapy of metabolic complications encountered during HIV infection. clin Pharmacokinet 41(14): 11951211.
- Arayne MS, Sultana N, Hashim MZ, Haroon U (2010) In Vitro Studies of Interaction between Metformin and NSAIDs (Non-Steroidal Anti-inflammatory Drugs) Using Spectrophotometry and RP-High Performance Liquid Chromatography. J Chil Chem Soc 55 (2): 206-211.
- Arayne MS, Sultana N, Nawaz M (2014) Investigation of Levocetirizine with HMG-CoA Reductase Inhibitors. Mod Chem Appl 2(3): 1000134.
- Xiangping L, Yingxiang D, Wen S, Junping K, Boyang Y (2009) SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy. 5(74): 1189-1196.
- Arayne MS, Najma S, Urooj H, Batool Z (2009) In Vitro Evidences For Simvastatin and Losartan Potassium Interaction and Its In Vivo Implications. J Chil Chem Soc 54(4): 432-436.
- Agha ZM, Arayne MS, Najma S, Qureshi F (2013) Spectroscopic Study To Characterize In Vitro Interaction of Losartan with Gliquidone and Pioglitazone. Medicinal Chemistry Research 22(1): 351-359.






























