Pharmacogenetics and Chemotherapy-Associated Hepatotoxicity in Children
Ocete-Hita E*, Urrutia-Maldonado E, Alés Palmer ML, Trujillo L and Jose M Gómez Luque
Hospital Universitario Virgen de las Nieves, Universidad de Granada, Spain
Submission: February 28, 2017; Published: March 27, 2017
*Corresponding author: Ocete-Hita E, Hospital Universitario Virgen de las Nieves, Universidad de Granada, URB. LOS GIRASOLES C/RIBERA 2, Villa de Otura, Granada, España, 18630, Granada, España, Tel: 630986936; Email: estherocete@ugr.es
How to cite this article: Ocete-Hita E, Urrutia-Maldonado E, Ales P M, Trujillo L, Jose M G L. Pharmacogenetics and Chemotherapy-Associated Hepatotoxicity in Children. J of Pharmacol & Clin Res. 2016; 2(4): 555592. DOI: 10.19080/JPCR.2017.02.555592
Abstract
Research findings in pharmacogenetics have shown that the response to chemotherapy is largely determined by genetic polymorphisms. Thus, children with the same type of tumour given the same type of treatment may present different responses, in terms not only of effectiveness but also of toxicity. We analyse recent studies of genetic polymorphisms associated with the use of chemotherapy in children and the development of hepatotoxicity, highlighting the importance and generalised use of methotrexate
Keywords: Chemotherapy; Pharmacogenetics; Hepatotoxicity; Leukaemia; Drug toxicity; Adverse drug reactions; Paediatric oncology
Abbreviations: 6-MP:6-Mercaptopurine; ALL: acute lymphoblastic leukaemia; 6-TGN 6-thioguanine; 6-MMPN: 6-methylmercaptopurine nucleotide; TPMT: thiopurine methyltransferase; ITPA: Inosine Triphosphate Pyrophosphatase; RBC: red blood cells; MTX: Methotrexate; ALT: Alanine Aminotransferase;MTHFR: Methylenetetrahydrofolate reductase
Method
A systematic review of the relevant scientific literature was conducted to compile and analyse the information currently available on pharmacogenetics and cancer hepatotoxicity in children.
Search strategy
Research papers were identified through a focused search of major scientific databases. The search terms used were "cancer, children, pharmacogenetics". The search was conducted in Pub Med via MeSH, Embase (until 16 December 2014) and Cochrane Central. All references retrieved were processed in Ref Works. The studies selected referred only to human subjects, male and female, in populations aged 0-18 years. When the terms "pharmacogenetics, chemotherapy" were entered as Title, Abstract or Keywords in the Cochrane Database of Systematic Reviews 21 clinical studies.
Introduction
Pharmacogenetics is the study of the pharmacological response of the individual, analysed by genotype. This science concerns our knowledge of the influence of heredity on individual variations in drug response, in terms of effectiveness and of the occurrence of adverse effects. Therefore, it concerns not pharmacokinetics but rather Pharmacodynamics [1]. With the rapid expansion of the range of antineoplastic drugs, new toxicities associated with their use have appeared, often in fields with many unknowns. The possibilities are many and not always predictable, and concepts such as idiosyncratic toxicity, overdose and cumulative effect must be differentiated [2]. The main toxic effect of these drugs is hepatotoxicity. There are no specific biomarkers enabling diagnostic certainty, and so the only way to diagnose hepatotoxicity secondary to the use of drugs is from clinical suspicion and by excluding other causes. This complicated outlook explains why the problem is such a challenging one [3]. Some patients with liver damage are clinically asymptomatic, while others present acute hepatitis or thrombosis. In most cases, liver damage in children is reversible and does not have a fatal outcome [4].
Recently-described genetic associations with hepatotoxicity
Many studies have been made of genetics and cancer pathology, mainly with respect to leukaemia (which is the most prevalent form among children). The aims of these studies are, on the one hand, to identify the genes associated with leukaemia, and on the other, to determine relations between genetic susceptibility and particular chemotherapeutic agents. The cytochrome P450 (CYP) 3A is very significant in this respect, for several reasons [5]. First, its CYP3A4, CYP3A5, CYP3A7 and CYP3A43 members are responsible both for the oxidation of organic substances such as lipids or steroids and for the metabolism of various toxic drugs. Second, CYP3A participates in the activation of cyclophosphamide and in the elimination of glucocorticoids and vincristine, drugs that are used in the treatment of acute lymphoblastic leukaemia. The following are some of the associations between hepatotoxicity and specific chemotherapeutic agents.
A. Mercaptopurine
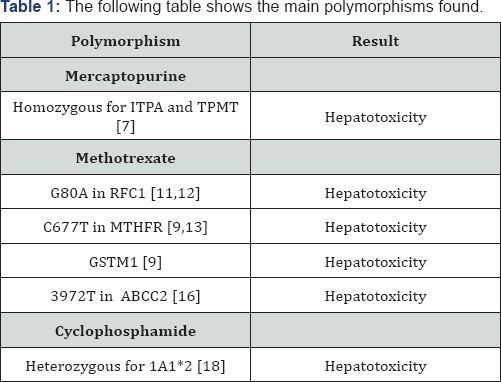
6-Mercaptopurine (6-MP) is currently employed as a key drug in the maintenance phase of acute lymphoblastic leukaemia (ALL), inhibiting de novo purine synthesis [6]. Its effectiveness is assisted by the active metabolites involved, namely 6-thioguanine [6-TGN] and 6-methylmercaptopurine nucleotide (6-MMPN). The pharmacogenetic polymorphism of thiopurine methyltransferase (TPMT) and of inosine triphosphate pyrophosphatase (ITPA) is associated with the individual metabolism by each subject of 6-mercaptopurine and with individual susceptibility to the development of hepatotoxicity. Specifically, the interindividual variability of levels of 6-TGN and 6-MMPN in red blood cells (RBC) is known to influence the efficacy of the drug. Accordingly, there is a high risk of myelo suppression among monozygotic TPMT-deficient patients. Other nucleoside polymorphisms have also been associated with liver damage. A recent study was conducted to determine the effectiveness of chemotherapeutic agents and possible associated liver damage according to the pharmacogenetic polymorphism of each subject [7].
For this study, 66 children with LLA and presenting diverse polymorphisms were chosen, and the metabolite levels in each subject, namely the individual genotypes of TPMT and ITPA, were analysed. The following findings were reported:
- 6-TGN concentrations in RBC are age-dependent and TPMT genotype-dependent.
- . 6-MMPN concentrations in RBC depend on the multilocus genotypes TPMT and ITPA 94 C>A. Patients with the ITPA variant and the original TPMT present higher concentrations of 6-MMPN.
- High concentrations of 6-MMPN in RBC are associated with liver toxicity: concentrations above the threshold of 4884 pmol/ 8 x 108 are predictors of risk of hepatotoxicity, with a positive predictive value of 95.7%. The hepatotoxicity observed at concentrations below this threshold was due to reductions in the dose of 6-MP or to treatment interruptions that led to its prolongation.
In fact, heterozygous patients with TPMT were observed to suffer more treatment interruptions than patients with the original genotype.
B.Methotrexate
Methotrexate (MTX) was one of the first drugs to be used in cancer treatment, and has proved valuable both as monotherapy for malignant trophoblastic disease and as a protagonist in treatment for leukaemia. MTX toxicity is dose dependent, and so the dosage used varies depending on the condition to be treated [8]. Most physicians reserve the term high-dose MTX for doses >500 mg/m2. The main forms of toxicity provoked are elevated transaminases and renal failure.
I. Methotrexate and hepatotoxicity: Hepatotoxicity provoked by methotrexate can occur with any dose range, and may present in various forms, including elevated transaminases, fibrosis and, at high doses (>1.5-2 g), cirrhosis. High doses of methotrexate can elevate transaminases from twice normal value to twenty times normal value, even in patients with folic acid rescue. Transaminase elevation occurs in 60-80% of patients and in most cases resolves spontaneously within a week or two. If alanine aminotransferase (ALT) does not fall below 180IU/l the dose should be reduced in the next cycle or even postponed. Elevated bilirubin levels normally fall in a few days, and the dose of MTX need not be reduced unless bilirubin exceeds 3mg/dl [6]. Very rarely, children with leukaemia who are treated with intravenous MTX are affected by liver fibrosis or hepatoma may appear.
II. Pharmacogenetics associated with the use of methotrexate [9]: A study was conducted at Kobe University Hospital, Japan, of 26 patients with acute lymphoid leukaemia, to investigate the association between certain polymorphisms and the occurrence of hepatotoxicity following treatment with methotrexate. GSTT1, GSTM1, GSTP1, RFC1, MTHFR and BCRP were genotyped, and the relation of each one with the appearance of hepatotoxicity was determined. RFC1 (reduced folate carrier) is expressed ubiquitously in the human body [10]. It is encoded by a gene located on the long arm of chromosome 21 (21q22.2-22.3) genes and, as the name suggests, it encodes a membrane protein called reduced folate carrier protein (Gene ID: 6573). Methotrexate is a substrate for this protein and its tissue distribution appears to be mediated by RFC1. RFC1 levels and the genetic polymorphism of RFC1 G80A [11] have been associated with treatment outcomes when LLA is applied, and also with methotrexate levels in these patients. Studies of the relationship between G80A polymorphism in RFC1 and the appearance of hepatotoxicity when high-dose methotrexate is administered have reported a positive association, suggesting that RFC1 is a key enzyme, determining the residual quantity of methotrexate in the liver.
According to these studies, a greater residual amount of methotrexate is present in the liver of patients with allele A at position 80 in RFC1, thus increasing the risk of hepatotoxicity [9]. In another recent study, of 500 children, it was found that the RFC 80AA variant was correlated with a lower risk of relapse (50% greater possibility of cure) compared with the GG and GA variants (p=0.046). It was also observed that haematologic toxicity was greater when high dose methotrexate was received by patients with the AA variant, with respect to the GA and GG variants. On the other hand, the increase in liver enzymes was greater in patients with the GG variant [12]. Methylenetetrahydrofolate reductase (MTHFR) is known to be a key factor in folate metabolism. MTHFR C677T genetic polymorphism has been linked with methotrexate hepatotoxicity in bone marrow transplant patients; this association has also been observed in patients with rheumatoid arthritis or ovarian cancer [13]. Previous studies have reported an association between MTHFR C677T and the appearance of neutropaenia, mucositis and anaemia in patients with ovarian cancer. Consideration of these results and of those reported in the present study suggests that the MTHFR genotype may be associated with the occurrence of hepatotoxicity, although further studies are needed to confirm this.
In fact, statistically significant results were obtained between being a carrier of MTHFR C677T and the residual dose of MTX at 48h (the dose associated with an increased risk of toxicity), and thus it seems logical that in patients with this polymorphism, rescue with leucovorin should be intensified. The results also show that being a carrier of GSTM1 increases the risk of hepatotoxicity, not only with the use of methotrexate, but also with that of acetaminophen [14]. Another gene associated with methotrexate is the protein-coding gene SLCO1B1 (solute carrier organic anion transporter family member 1B1). A statistically significant association has been found between the genotype rs11045879 CC and high concentrations of MTX in plasma. In consequence, it is possible to predict which patients are at greater risk of toxicity [15]. Another allele that has been associated with hepatotoxicity is the polymorphic 3972T variant of the gene ABCC2; in this respect, a statistically significant inverse relationship has been described [16]. Research into other genetic polymorphisms related to plasma concentrations of methotrexate and toxicity continues, for example with respect to the genes ARID5B and SLC22A8 [17].
C. Cyclophosphamide
Cyclophosphamide is an oxazaphosphorine DNA alkylating agent that is currently used in the treatment of many malignant diseases, both in children and in adults. It is a prodrug which enters the liver and is metabolised by the hepatic cytochrome P450 system into active and inactive components. Oxidation in the C4 position generates 4-hydroxycyclophosphamide, in a reaction that is mediated by various isoforms, including CYP 2A6, 2B6, 2C8, 2C9, 2C19, 3A4 and 3A5. After several reactions, this produces phosphoramide mustard, the anti-tumour active form, and acrolein, the metabolite responsible for urotoxicity [18]. With respect to the polymorphism of aldehyde dehydrogenase and the effects of cyclophosphamide, it has been observed that patients who are heterozygous for 1A1*2 present an increased incidence of liver toxicity. On the other hand, patients who are heterozygous for 3A1*2 present a higher incidence of haemorrhagic cystitis. Table 1 summarise the main associations found.
Conclusion
Children with the same type of tumour and who are given the same treatment can present different clinical responses, in terms of efficacy and of drug toxicity, which suggests there may be individual genetic factors that influence the occurrence of side effects such as hepatotoxicity. In this study, we examine hepatotoxicity and its possible relationship with genetic factors with particular respect to the use of methotrexate. However, many other studies have suggested different associations between polymorphisms and treatment response. Therefore, further research should be aimed at minimising toxicity and at obtaining personalised treatments according to the individual risk of toxicity presented by each patient. The following table shows the main polymorphisms found.
References
- Dauden Tello E (2006) Pharmacogenetics I. Concept, history, objectives and areas of study. Actas Dermosifiliogr 97(10): 623-629.
- Floyd J, Mirza I, Sachs B, Perry MC (2006) Hepatotoxicity of chemotherapy. Semin Oncol 33(1): 50-67.
- Hita EO, Garcia JA, Gonzalez JC, Molina AA, Cordero MA, et al. (2012) Amoxicillin-clavulanic acid hepatotoxicity in children. J Pediatr Gastroenterol Nutr 55(6): 663-667.
- Ocete Hita E, Martin Garcia JA, Gimenez Sanchez F, Flores Gonzalez JC, Abril Molina A, et al. (2013) Hepatotoxicity due to drugs or natural products in children. An Pediatr (Barc) 78(4): 248-259.
- Aplenc R, Glatfelter W, Han P, Rappaport E, La M, et al. (2003) CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. Br J Haematol 122(2): 240-244.
- Green MR, Chowdhary S, Lombardi KM, Chalmers LM, Chamberlain M (2006) Clinical utility and pharmacology of high-dose methotrexate in the treatment of primary CNS lymphoma. Expert Rev Neurother 6(5): 635-652.
- Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, et al. (2011) Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol 71(4): 575-584.
- Erculj N, Kotnik BF, Debeljak M, Jazbec J, Dolzan V (2012) Influence of folate pathway polymorphisms on high-dose methotrexate-related toxicity and survival in childhood acute lymphoblastic leukemia. Leuk Lymphoma 53(6): 1096-1104.
- Imanishi H, Okamura N, Yagi M, Noro Y, Moriya Y, et al. (2007) Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet 52(2): 166-171.
- Niedzielska E, Weclawek-Tompol J, Matkowska-Kocjan A, Chybicka A(2013) The influence of genetic RFC1, MS and MTHFR polymorphisms on the risk of acute lymphoblastic leukemia relapse in children and the adverse effects of methotrexate. Adv Clin Exp Med 22(4): 579-584.
- Laverdiere C, Chiasson S, Costea I, Moghrabi A, Krajinovic M (2002) Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood 100(10): 3832-3834.
- Gregers J, Christensen IJ, Dalhoff K, Lausen B, Schroeder H, et al. (2010) The association of reduced folate carrier 80G>A polymorphism to outcome in childhood acute lymphoblastic leukemia interacts with chromosome 21 copy number. Blood 115(23): 4671-4677.
- Toffoli G, Russo A, Innocenti F, Corona G, Tumolo S, et al. (2003) Effect of methylenetetrahydrofolate reductase 677C-->T polymorphism on toxicity and homocysteine plasma level after chronic methotrexate treatment of ovarian cancer patients. Int J Cancer 103(3): 294-299.
- Prescott DM, Dizick SJ (2000) A unique pattern of intrastrand anomalies in base composition of the DNA in hypotrichs. Nucleic Acids Res 28(23): 4679-4688.
- Lopez-Lopez E, Martin-Guerrero I, Ballesteros J, Pinan MA, Garcia- Miguel P, et al. (2011) Polymorphisms of the SLCO1B1 gene predict methotrexate-related toxicity in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 57(4): 612-619.
- Sharifi MJ, Bahoush G, Zaker F, Ansari S, Rafsanjani KA, et al. (2014) Association of -24CT, 1249GA, and 3972CT ABCC2 gene polymorphisms with methotrexate serum levels and toxic side effects in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol 31(2): 169-177.
- Csordas K, Lautner-Csorba O, Semsei AF, Harnos A, Hegyi M, et al.(2014) Associations of novel genetic variations in the folate-related and ARID5B genes with the pharmacokinetics and toxicity of highdose methotrexate in paediatric acute lymphoblastic leukaemia. Br J Haematol 166(3): 410-420.
- Pinto N, Ludeman SM, Dolan ME (2009) Drug focus: Pharmacogenetic studies related to cyclophosphamide-based therapy. Pharmacogenomics 10(12): 1897-1903.






























