CR845 (Difelikefalin), A Kappa Receptors Agonist In Phase III By CARA Therapeutics: A Case Of 'Spin' In Scientific Writing?
*Jan M Keppel Hesselink
Department of Molecular Pharmacology, University Witten/Herdecke, Germany
Submission: February 08, 2017; Published: March 10, 2017
*Corresponding author: Jan M Keppel Hesselink, Department of Molecular Pharmacology, University Witten/Herdecke, Germany, Email:jan@neuropathie.nu
How to cite this article: Jan M Keppel Hesselink. CR845 (Difelikefalin), A Kappa Receptors Agonist In Phase III By CARA Therapeutics: A Case Of 'Spin' In Scientific Writing?. J of Pharmacol & Clin Res. 2017; 2(3): 555588. DOI: 10.19080/JPCR.2017.02.555588
Abstract
Maintaining high standards of scientific integrity in communications related to drug development is of utmost importance, as it helps to maintain public trust and investors trust in the research enterprise. Here we will analyze the structure of scientific company communications during nearly a decade, related to the peripherally acting kappa receptors agonist CR845 (difelikefalin). This drug is currently developed by Cara Therapeutics. It forms a new principle to treat chronic or post-operative pain by a peripheral acting opioid, which is supposed to be devoid of central opioid side effects. Phase I development of CR845 started in 2008, phase II studies have been completed, and phase II/III is ongoing for two formulations: an intravenous formulation for postoperative analgesia and uremic pruritus, and an oral formulation for treatment of chronic pain such as in osteoarthritis. Soon (1st half of 2017) new data from phase IIb is expected to become available. It is scheduled that the file of CR845 will be submitted to the authorities for approval in 2018; the key indication for the IV formulation is still unclear.
Although the clinical development phase started in 2008, primary scientific data on CR845 in peer reviewed journals to date are absent. The only sources for information and valuation of the company available are some abstracts and posters, written by company employees, and many press releases of CARA Therapeutics, presenting results of research and development studies. All scientific information related to CR845 after 9 years of development thus are company based and drive the valuation of the company on the stock market. We will present the profile of CR845 based on the information presently available and discuss this in the context of disseminating scientific information in a peer review free environment. So far the company's track record of quick recruitment in trials and the promising profile communicated supports hope for the targeted indications. Our recommendation for companies developing new treatment principles is, to facilitate actively publications of data in peer reviewed journals as soon as possible. Press releases should not stay long in the public domain as the sole vehicles for the communication of new scientific facts and the sole base of the valuation of the company and its assets.
Abbreviations: EMA: European Medicines Agency; PIDs: Pain Intensity Differences; HAL: Human Abuse Liability
Introduction
How to maximize the economic and social benefit, without loss of scientific integrity? A question put forward in the publication 'Big Science [1]: What’s It Worth? From CERN, ESADE Business School and the Aalto University, but remained a question with no answer. Maintaining high standards of ethical and scientific integrity is of utmost importance, as it helps to maintain public trust and investors trust in the research enterprise [2]. The integrity of a scientist should be based on adherence to two core values of science: respect for evidence and evidence-sharing [3]. We will analyze respect for evidence and evidence-sharing based on what is known about CR845 in the public domain, press releases of CARA and some posters presented by company representatives, based on the context we outline here. It is known, that under the US securities laws (Securities Act of 1933), company are required to publish results, which an average investor would deem important in making investment decisions, as soon as possible. However, there is great freedom in how this public reporting obligation can be shaped, and the case of CR845 will serve as an example, based on which we will make some recommendations.
Peer Reviewed Data
It is generally felt, that scientific data presented in peer reviewed, Pub Med indexed journals have a higher chance to be reliable compared to data published elsewhere. If company employees are the sole authors of a paper, peer review is an important tool to ensure scientific integrity [4]. This instrument is absent if companies communicate their data via press releases. In such cases, the information disseminated needs the optimal attention of the company to ensure credibility and reliability of its content, in the context of scientific integrity. We will analyze the case CR845 against this background. Company statements will be presented as verbatim quotes, in order to be able to be precise in our analysis of how the authors shaped the impression their results produce in readers, characterized by Fletcher and Black in 2007 as 'spin' in scientific writing [5]. Richard Horton in his landmark paper in the BMJ of 1995 already pointed out how the authors used "their power as owners of their writing to emphasize one point of view more than another [6]." Furthermore, we will look for 'hedging' or 'cautious language' in the various press releases. Hedging in formal academic writing is regarded as a way for the scientist to establish academic integrity [7]. Recently Ter Riet and colleagues analyzed a great number of scientific texts, and found that publications on industry-supported studies used significantly fewer hedges than publications not so supported (p = 0.028) [8].
Peripheral Kappa receptor agonists: the scientific and development context
There is an ongoing intense debate about pros and cons of prescription of opioids. Without taking a stance in this discussion, it is clear that less or non-addictive opioids would be greatly welcomed. Peripherally acting opioid agonists may be such analgesics. Loperamide hydrochloride is a peripherally acting opioid agonist, which had analgesic action in pain models; its action is based on its affinity for the delta-opioid receptor as it can be antagonized by naltrindole, a delta-opioid receptor selective antagonist [9]. However, there are some indications for the development of tolerance [10]. The rationale of kappa receptor agonist with limited ability to penetrate the CNS is outlined already in 1994 [11].
The kappa agonist as imadoline (EMD-61753) is currently in development phase III by Tioga Pharmaceuticals for the indication: pruritus associated with atopic dermatitis. Naloxegol belongs to the same class of peripheral kappa agonists, and is available since 2014 in the USA as a once-daily oral therapy indicated for the treatment of opioid-induced constipation. The chemical principle to create kappa agonists, restricted to the peripheral compartment is based on making the compounds hydrophilic, for instance by glucuronidation, as inmorphine-6- glucuronide, arylacetamide (ADL 10-0101), TRK-820, HS-731 and triazaspiro, or to create peptidergic compounds, such as D-Arg2, Lys4-Dermorphin-(1-4)-amide (DALDA), FE200665, CR665 and CR845 [12]. CR845, a kappa receptors agonist and a D-tetrapeptide analogue, developed by Cara Therapeutics, originating from the Swiss company Ferring Pharmaceuticals, belongs to the class of peripherally acting kappa agonists. The compound is also known as CKD-943, FE 202845 and MR13A9. Its history dates back to 2005, when a prototype of a kappa receptors agonist under the code name FE200665 (CR 665), designed by Ferring, completed phase Ia clinical trials in that year. This compound was a D-amino-acid tetrapeptide, well tolerated in humans, with no reports of dysphoria or hallucinations, and as effective as oxycodone in a human model of acute visceral pain [13].
However, CARA indicated in the poster on the preclinical profile of CR845 the compound was not orally bioavailable. CR845 was positioned as a second-generation peptide, orally bioavailable, and CARA reported to have completed Phase I clinical trials in 2009 [14]. CARA is said to own six patents related to CR845, with claims covering compositions of matter and methods of use for CR845. The earliest patent claiming CR845 compositions will expire in 2027 [15]. The company website of Cara Therapeutics claims for the compound 'AN UNPRECEDENTED LEVEL OF SELECTIVITY FOR KAPPA RECEPTORS LOCATED ON PERIPHERAL NERVES’, and the quote is verbatim, in capitals [16]. CR845 as an intravenous formulation is in phase II/III development for postoperative pain and uremic pruritus. The oral formulation for the treatment of chronic pain is in phase II for chronic pain. The website of the company highlights CR845 kappa receptor selectivity compared to previously developed kappa agonists such as enadoline, asimadoline, and TRK-820; these 3 compounds are characterized by a less than 5000-fold selectivity for the kappa receptor, while CR854’s selectivity is said to be at least 6 times as high, without any significant affinity for any other non-opioid known receptor. TRK-820 from Toray Industries, Inc, also known as nalfurafine, is available in Japan and registered since 2009 for the treatment of uremic pruritus, one of the current development indications for CR845 IV [17].
The compound has been designated as an orphan drug by the (European Medicines Agency EMA) for this indication. In an educational video at the company's website, CR845 is positioned as a high selective peripheral acting kappa receptor agonist, binding to nerves and immune cells in the periphery, and blocking in pain and inflammation, without the side effects of current opioids. The profile presented, as well as the company’s track record of fast recruitment in clinical trials, seen by many as a predictor for success, supports hope for the near further future of uremic patients suffering from pruritus, where no standard treatment exists. Whether pricing issues of the IV formulation later on will ask for the post-operative indication to be licensed out, or sacrificed, remains to be seen.
Publications on CR845 in peer reviewed articles?
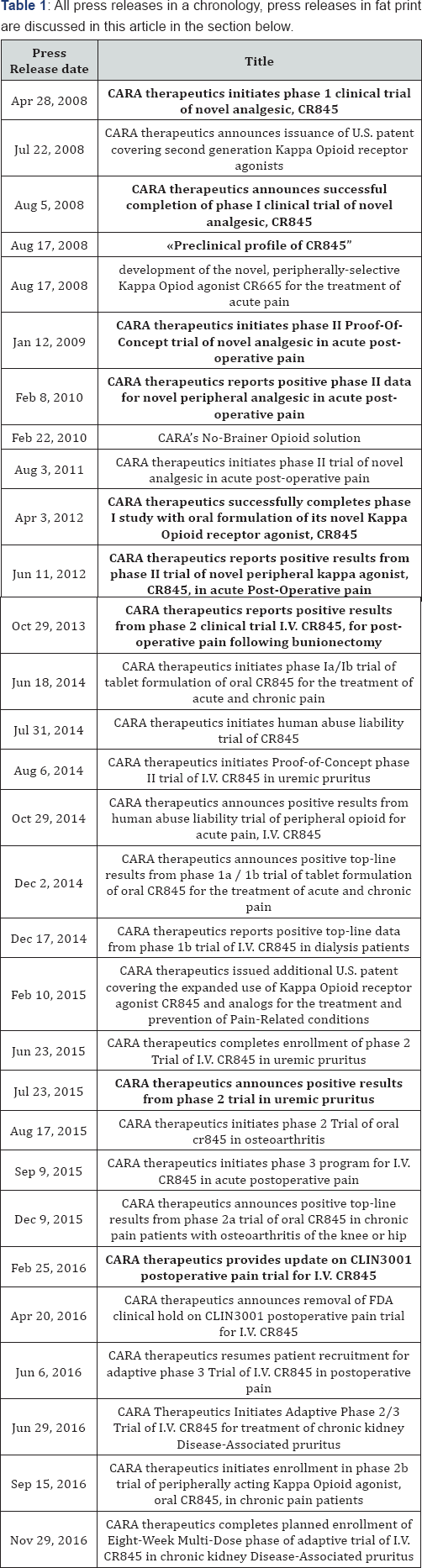
We could not find any full publication about the effects of this drug in peer reviewed journals, only 3 published posters [18-20]. In addition we identified two book chapters (the same chapters, written by the same authors), published in 2015, without much detail [21]. Authors of all posters were employees of CARA. In the first poster data were presented from an unblinded, pooled treatment-emergent adverse events analysis, based on in a total of 368 patients, from 3 double-blind, randomized, placebo- controlled, phase 2 clinical studies. In the second poster, the preclinical profile is presented (see under), and the last poster, presented the results of a phase II bunionectomy study. Although the goal of the study reported in the first poster was said to be side effect reporting, the abstract was mainly focused on efficacy data, and the conclusion was: "These results suggest that CR845 not only provides post-operative analgesia but also appears to reduce Post-Operative Nausea and Vomiting, an effect that may not be solely related to a reduction in mu opioid rescue use." This conclusion seems to overstretch the initial goal of the analysis a bit. It has already previously been our recommendation to biotech and pharmaceutical industries not to publish results of scientific studies, without a clear back up of the same results by peer reviewed papers as soon as possible [22,23]. On the website of CARA, we can find a great number of detailed and high quality press releases related to CR845, the first press release dates of April 2008 and announced the start of the phase I program ( Table 1). CARA has put quite some effort in these press releases, and the website as well as the index for press releases and the releases proper are all of high quality.
Preclinical profile
The preclinical profile has been published as a company poster: 'Preclinical Profile of CR845: A Novel, Long-Acting Peripheral Kappa Opioid Receptor Agonist', presented at the IASP in 2008 [19]. The compound was presented as a highly selective for both human and rodent KOR, without off-target activity tested in 94 receptors-, channels- and transporters assays. Ki was reported to be 0.32 nM for the hKOR, and only >10000 for the hMORenhDOR. In various pain paradigms CR845 dose dependently inhibited pain behavior. In the 0.6% acetic acid induced writhing in male CD-1 mice 0.01, 0.03, 0.1 and 0.3 mg/kg IV were tested versus vehicle and ED50 was estimated on 0.07 mg/kg IV after 15 minutes exposure. In an abdominal pain model, DBTC-induced pancreatitis and in the CFA-induced inflammatory pain ED50 was 0.3 mg/kg IP or IV. In the 2% carrageenan intraplantar model 0.3 and 1 mg/kg were significantly better than vehicle. TNF-alpha could be reduced in a sepsis model by 47% by CR845 10 mg/kg (prednisolone 3 mg/ kg 64%). In the neuropathic pain Chung model ED50 was 0.38 mg/kg IV. In an itch model, 48/80 or 5'GNTI induced pruritus, CR845 0.1 and 0.3 mg/kg significantly inhibited scratch behavior, ED50 = 0.08 mg/kg IV.
CR845 studies in clinicaltrials.gov
In order to be able to analyze the completeness of the company reporting its results, it is relevant to summarize the 8 clinical trials registered for the compound in clinicaltrials.gov:
A. Completed studies
I. Phase II study
ClinicalTrials.gov Identifier: NCT01361568.
A multi-center, double-randomized, double blind, placebo controlled study to evaluate the analgesic efficacy and safety of intravenous CR845 dosed preoperatively and postoperatively in patients undergoing a laparoscopic hysterectomy; n=203.
Period: 2011-2014.
Dose and treatment duration: Single i.v. dose (0.04 mg/kg) administered pre-/ or postoperatively for pain versus placebo.
Reported in CARA Press release.
II. Phase II study
ClinicalTrials.gov Identifier: NCT01789476.
A single-center, randomized, double-blind, parallel group, placebo-controlled study to evaluate the analgesic efficacy and safety of cr845 dosed in patients with pain following bunionectomy surgery; n=51.
Period: 2013-2015.
Dose and treatment duration: 0.005 mg/kg per dose, IV bolus. The initial dose was administered upon reaching a qualifying pain intensity score and followed by a supplemental dose, if requested by patient for pain. Additional doses could be administered every 8 hours up to 48 hours.
Reported in CARA Press release.
III. Phase II study
ClinicalTrials.gov Identifier: NCT00877799.
A Phase 2, randomized, double-blind, placebo-controlled, proof of concept study to evaluate the analgesic efficacy and safety of intravenous cr845 during the post-operative period in subjects undergoing laparoscopic-assisted hysterectomy; n=114.
Period: 2009-1015.
Dose and treatment duration: CR845 15-min i.v. infusion at doses of 0.008 or 0.024 mg/kg on the day after surgery (Cohort 1), or at a dose of 0.040 mg/kg immediately after surgery (Cohort 2) versus placebo.
IV. Phase II study
ClinicalTrials.gov Identifier: NCT02524197.
A study of the safety and a single-blind, multiple ascending- dose pilot study of the safety, tolerability, pharmacokinetics, and effectiveness of orally administered cr845 in patients with osteoarthritis of the hip or knee; n=81.
Period: 2015-2016
Dose and treatment duration: Oral administration of CR845 0.25 mg, 0.50 mg, 1 mg and 5 mg tablets; 38 days
Reported in CARA Press release
V. Phase II study
ClinicalTrials.gov Identifier: NCT02229929.
A double-blind, randomized, placebo-controlled study to evaluate the safety and pharmacokinetics of intravenous cr845 in hemodialysis patients, and its safety and efficacy in hemodialysis patients with uremic pruritus; n=89.
Period: 2014-1016.
Dose and treatment duration: Intravenous CR845, 0.5 mcg/ kg; 1.0 mcg/kg; 2.5 mcg/kg versus placebo; after each dialysis session over a 2 week treatment period (3 times per week).
Reported in CARA Press release.
B. Running studies
I. Phase II
ClinicalTrials.gov Identifier: NCT02944448.
A randomized, double-blind, placebo-controlled, titration- to-effect study of orally administered CR845 in patients with osteoarthritis of the hip or knee; n=330.
Period: 2016-2017.
Dose and treatment duration: 1; 2.5; 5 mg bid versus placebo during 8 weeks.
First Results expected 2017.
II. Phase II/III
ClinicalTrials.gov Identifier: NCT02858726.
A two-part, multicenter, double-blind, randomized, placebo-controlled study to evaluate the safety and efficacy of intravenous cr845 in hemodialysis patients with moderate-to- severe pruritus; n=400.
Period: 2016-2018.
Dose and treatment duration: CR845 0.5 mcg/kg, 1 mcg/ kg, 1.5mcg/kg versus Placebo for 12 weeks in patients on hemodialysis.
First Results expected 2017.
III. ClinicalTrials.gov Identifier: NCT02542384
A multicenter, randomized, double-blind, placebo-controlled, adaptive design study evaluating the analgesic efficacy and safety of intravenous cr845 in patients undergoing abdominal surgery; N=450.
Period: 2015-2017.
Dose and treatment duration: CR845 IV (0.5 or 1 mcg/kg) or matched placebo, every 6 hours during the 24-hour postoperative treatment.
First Results expected 2017
It is interesting to find the hysterectomy not explored in phase III, while the company did not report the results of phase II in any of their press releases.
IV. Press releases of CARA
We will first summarize all press releases, in so far as directly related to CR845, and continue highlighting some important scientific facts as presented in the press releases, as our material for further analysis.
C. Early studies (2008-2012)
Apr 28, 2008 CARA announced the initiation of its IV phase I program, and defined the compound as: "long-acting peripheral kappa opioid receptor agonist". The argument for the qualifier 'long-acting’ was given by referring to animal models, were the compound "exhibits analgesic efficacy for up to 18 hours after a single dose." In the company poster on the results of the preclinical models however, published some months later, the analgesic effects were only described up to 8 hours after administration [19]. On August 5, 2008, the results of a phase I study, evaluating the IV formulation, were presented at the company's website as a press release [24]. It was a study evaluating the safety, tolerability, pharmacokinetic profile, and pharmacological activity of CR845 in a double-blind, randomized, placebo-controlled, single escalating intravenous dose study in 54 healthy male and female volunteers. The study was not registered in clinicaltrials.gov. The statement of the company was: "Linear, dose-proportional increases in systemic exposure to CR845 were observed. Low doses of CR845 resulted in plasma levels at or above the plasma levels of drug expected to be associated with clinical analgesic efficacy. CR845 infusion triggered a quantitative endocrine biomarker of peripheral kappa opioid receptor activation at the lowest dose tested." Phase I data of the oral formulation were published on the company’s website on April 3, 2012, and highlighted a: "- robust bioavailability and pharmacologic activity seen with oral formulation of peptide-based Kappa opioid agonist" [25] - Pharmacologist in general would have difficulty to understand what is meant by "robust bioavailability".
On the company website data are presented of a phase I study in 50 male volunteers randomized to placebo or one of four single ascending doses of an enteric-coated, capsule formulation of CR845. Mean oral bioavailability was reported to be 16% in all groups, with peak and total exposures proportional to each dose. The compound was reported to be safe and generally well tolerated across all doses tested. The VP R&D added to the data "We are very impressed with the bioavailability and bioactivity exhibited by this oral formulation of CR845." The initiation of the first phase II Proof of Principle study was announced in a press release, dated June 12, 2009. 120 patients would be enrolled, "randomly selected for treatment with one of two doses of CR845 or placebo." This most probably refers to the first phase II study defined in clintrials.gov as study NCT01361568, defined as a study in 203 patients, and first received on April 6, 2009. Dose regime of this study was CR845 administered as a single 15-min i.v. infusion at doses of 0.008 or 0.024 mg/kg on the day after surgery (Cohort 1) or at a dose of 0.040 mg/kg immediately after surgery (Cohort 2). On February 8, 2010, the company announced: "Positive Phase II Data for Novel Peripheral Analgesic in Acute Post-Operative Pain" The study was defined as: "The 46 patient Phase II, multi-center, double-blind, placebo- controlled study was conducted at eight hospitals" Patients were dosed either with placebo or 0.040 mg/kg CR845.
The results were defined as: "Significant pain relief was observed in CR845-treated patients over placebo from 4-8 hrs post-drug administration, as exemplified by a significant change in pain intensity difference (PID) scores (p<0.05)."Without any further context, it is difficult to understand how the planned n=203 patient study evolved in 120 patients- and subsequently in a 46-patient study. However, the data presented at clinicaltrials. gov seems to solve this mystery: in cohort 2 there were included 26 patients on placebo and 20 on CR845 0.040 mg/kg, dosed immediately post-op. (Day 0) [26]. Of these 46 patients, there were 3 non-completers: 1 protocol violation and 2 lost for follow up. The unsolved question of course is, whether the reported significant pain reduction was in the completer population or in the ITT population. Further phase II data in 'Acute Post-Operative Pain' have been presented by the company at the company's website on June 11, 2012 [27]. We could not identify any poster or paper to date presenting these data, in contrast with the later completed bunionectomy study, where the data were published in a poster, presented in 2015. In the communication from 2012, Cara Therapeutics highlighted "positive results from a Phase II study for the treatment of acute post-operative pain with its novel, peptide-based, peripherally-acting kappa opioid agonist, CR845". The Phase II study (NCT01361568) was a double-randomized, double-blind, placebo-controlled trial and evaluated one intravenous dose of CR845 (0.04mg/kg/BW) in women undergoing a laparoscopic hysterectomy. 203 patients were enrolled and randomized in four treatment arms:
- both a pre-and a post-operative dose of CR845;
- a single pre-operative dose of CR845;
- a single post-operative dose of CR845; and
- Both pre- and post-operative placebo.
The company stated that the study: "successfully met its primary endpoint, the reduction of the amount of rescue opioid analgesics used in a 24-hour period after surgery". Furthermore, CR845 reduce postoperative pain measured as pain intensity differences (PIDs) and summed pain intensity differences. Patients receiving both a pre- and postoperative dose of CR845 achieved the study's primary endpoint: a statistically significant reduction (33%, p<0.05) of morphine use over 24 hours compared to the placebo group (8 mg morphine less acc. to clintrials.gov). Significant 24-hour analgesic effects were also reported for various other measurements. Pre- and/or post-surgical dosing of CR845 was reported to be safe and well tolerated, and common opioid-related side effects of nausea, vomiting and pruritus were not frequently seen. The analysis described on clinicaltrials.gov refers to a total of 'Participants Analyzed' of 183. The chief medical officer of Cara therapeutics commented: "The results of this Phase II trial have further confirmed the robust analgesic efficacy of CR845 in this acute post-operative setting," and "these data have established the clear clinical advantage of dosing CR845 before and after surgery in significantly reducing the severity of patient pain and the need for opioid rescue medication in the first 24 hours post-surgery."
There were more interesting statements, one from the principle investigator, Professor of Anesthesiology Tong J. Gan: "These results indicate that this new class of anti inflammatory/ analgesic can potentially be used in a multimodal analgesic strategy, including preoperative dosing, to improve postoperative pain and reduce the need for morphine and other opioids." The hedge in the quote is underlined. And a company quotes: "This trial with CR845 has clearly demonstrated that a peripherally-selective kappa opioid agonist is a fundamentally new and viable option for the treatment of acute postop pain," from Derek Chalmers, CEO at Cara. We underlined 2 fragments as clearly strong statements and no hedges at al. The company summarized the status of the drug in the 2012 press release as:
- A highly selective, peptide-based, peripherally- restricted kappa opioid receptor agonist,
- in development for the treatment of acute and chronic pain and pruritus,
- developed as an intravenous (IV) formulation of CR845 for hospital use,
- Completed a successful Phase I study of an oral capsule formulation of CR845 aimed for clinical development for the treatment of postoperative pain following hospital discharge and chronic inflammatory pain.
- Safe and well tolerated in the more than 300 patients dosed, wit dysphoric reactions or hallucinations characteristic of centrally-acting, non-peptidic kappa opioid agonists.
D. More recent studies (2013-2016)
a. Results of the bunionectomy phase II study: October 29, 2013: the results of a phase II study in the treatment of acute pain following bunionectomy were presented by the company [28]. The company stated that in its press release "in a pre-specified analysis for patients who completed the trial ("Completer Analysis"), repeat dosing of I.V. CR845, over 48 hours post surgery, provided statistically greater pain reduction than placebo at both the 24 and 48 hour time points following initiation of treatment, as assessed using the FDA recommended endpoint, the Summed Pain Intensity Difference (SPID)."The treatment evaluated was IV 0.005 mg CR845/kg/dose versus placebo in 51 women and men undergoing a primary unilateral first-metatarsal bunionectomy surgery. Clearly the issue here raised is related to the ITT analysis, because by stipulating the results of the completer analysis, implicitly might suggested that the ITT analysis was not significant. In a later communication in 2015 however, the issue of completer analysis was disregarded. In 2015 the company stated: "In multiple randomized, doubleblind, placebo-controlled Phase 2 trials in patients undergoing laparoscopic hysterectomy or bunionectomy procedures, I.V. CR845 treatment resulted in statistically significant reductions in both pain intensity and opioid-related side effects [29]."
In addition, the company pointed out in 2014, that I.V. CR845 treatment resulted in a statistically significant reduction in the incidence of opioid-related adverse events of nausea and vomiting (by 60% and80%, respectively; p<0.05) compared to placebo during the 48-hour period of treatment. It was not clear whether these data were based on the ITT or on the complete analysis. The CEO summarized the positive phase II data in the 2 indications mentioned above and stressed "I.V. CR845's broad and robust analgesic potential." This all became somewhat more clear only after the publication of abstract 422 in the supplement of the Journal of Pain in 2015, written by the company employees: "CR845, a peripheral kappa opioid, provides better pain relief with less nausea and vomiting than placebo in patients after bunionectomy." The authors added in this poster that pain reduction in the intent to treat analysis did not reach significance for the primary efficacy measurement, the mean summed pain intensity differences from baseline over 24 hour. Referring to the significant effects in the complete analysis, the authors added: 'This observation was supported using the modified intent-to-treat population in which a greater decrease in SPID0-24 was observed with CR845 than placebo, although this difference was not significant (P=0.116).’ In clinicaltrials. gov we can see that the significance was calculated in n=40, while the total number of patients included was 51.
b. Results of the uremic pruritus phase II study: Press release of 23 July 2015: "Cara Therapeutics announces positive results from Phase 2 Trial in Uremic Pruritus". This study was a double-blind, randomized, placebo-controlled trial in 65 dialysis patients. (In clinicaltrial.gov this study was coded as NCT02229929, and 89 patients were scheduled for enrollment: in Part A of the study 3 different dosages and placebo were tested during a week: 0.5 mcg/kg; 1.0 mcg/kg and 2.5 mcg/ kg), in part B one dose (1.0 mcg/kg and placebo were tested for 2 weeks. The primary endpoint was defined as change from baseline of the average worst itching during the second week of treatment. Results according to the press release (no results entered in cinicaltrials.gov): patients on active drug experienced a 54 percent greater reduction in worst itch scores than those receiving placebo (p-value = 0.016.No details were given whether this 54% was obtained for instance in the highest dose group during the part A of the study, or in the medium dose group of the part B. Secondary endpoints focused on quality of life measures, and patients on active drug experienced significantly better QOL at the end the two-week treatment period than those receiving placebo (p-value=0.031). "The principle investigator, Professor Tumlin, from the University of Tennessee was quoted:" "With no approved therapy and the limited efficacy of current options, CR845 provides an opportunity to alleviate the pain and discomfort of this persistent clinical problem among ESRD patients." Strong language, no hedges, and interestingly he referred to pain and not to itch.
c. Results of the oral osteoartrosis phase II study: Press release December 9, 2015: Cara Therapeutics announces positive top-Line results from Phase 2a Trial of Oral CR845 in chronic pain patients with Osteoarthritis of the knee or hip. This press release was one of the more extensive ones. The Phase 2a study was a single-blind, randomized, multiple ascending dose trial in 80 patients, evaluating the oral CR845 tablets dosed over a two- week treatment period in patients experiencing moderate-to- severe pain from osteoarthritis of the knee or hip. Four strengths were tested: 0.25 mg, 0.5 mg, 1.0 mg and 5.0 mg, dosed twice a day during two-week. The mean joint NRS pain score exhibited a dose-related reduction from baseline to the end of the two-week treatment period, ranging from -25 percent at the lowest (0.25 mg) tablet strength up to -34 percent for the highest (5.0 mg) tablet strength.
More in detail: "50 percent of the patients in the 5.0 mg dose group reported at least a 30 percent reduction in their pain score at the end of the treatment period. Integrated AUC analysis of the overall NRS score for the entire treatment period indicated a statistically significant reduction in the 5.0 mg dose group compared to the three lower doses used in the trial (Wilcoxon Rank Sum Test: p=0.02). The reduction in pain score in the 5.0 mg dose group was accompanied by a statistically significant reduction in mean rescue medication of approximately 80 percent (ANOVA: p= 0.02, for 5.0 mg vs. lower dose groups)." Derek Chalmers, CEO: "These results are an important first step in establishing the applicability of an oral formulation of CR845 in treating chronic pain patients." The company also reported dose-proportional PK effects, and the 5.0 mg dose group showed an approximately five-fold increased mean AUC value compared to the 1.0 mg dose group. The Chief Medical Officer of Cara Therapeutics: "Although this Phase 2a study was designed primarily to confirm safety, tolerability and PK parameters in this challenging patient population, I'm very pleased that we obtained significant converging findings of dose-related effectiveness."
Consolidation of ‘facts' by independent authors
- It is a small peptide molecule,
- without penetration in the central nervous system ('A kappa agonist without kappa effects in the brain'),
- Effective against acute, post-surgical pain based on the results of a n=400 phase II study,
- Major side effects of CR845: facial tingling or numbness, dizziness and fatigue,
- Completed a human abuse liability (HAL) study with supportive results (n=40)
- The drug has no rewarding properties and doesn't have the negative effects of respiratory depression, and
- The drug is tested primarily as an intravenous drug for hospital use.
Opinion of financial analysts
Information on CR845 is currently the only driver and base for the opinion of analysts, advising life science investors. It is therefore of interest to shortly evaluating the advices of such analysts. The Nasdaq on February 1st, 2017 gave a 'strong buy' advice, based on 4 different analyst companies: H C Wainwright, Janney Mont, Piper Jaffray and Stifel Nicolaus. In 2017, the results of IV study in Uremic pruritus expected (itch), as well as clinical data on the 2 other indications. These events, based on the previous released information, clearly will influences the stock market, as we can see in Figure 1 of the Nasdaq chart; since 1-1-2017 the value rose from just under $10 to nearly $16 on February 1st, and given the clear 'buy' signals, together with the information of CARA, it is expected to raise even more. These events, based on the previous released information, clearly will influences the stock market, as we can see in Figure 1 of the Nasdaq chart; since 1-1-2017 the value rose from just under $10 to nearly $16 on February 1st. Given the clear 'buy' signals, together with the information of CARA, this recent share price gain is clearly a sign of investor's anticipation of potentially positive future data for CR845.
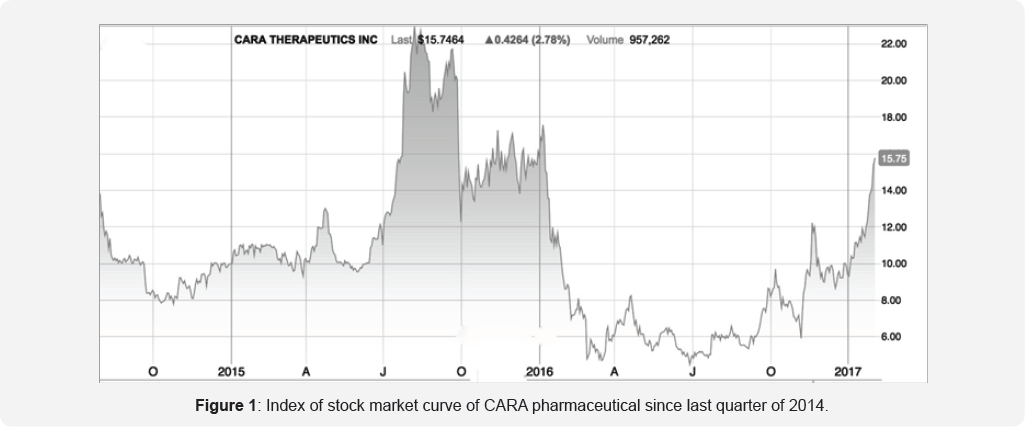
The stage was directly set in strong positive wording, without hedges, in one of the first communications on the results of the phase I data of the IV formulation from 2008, even low doses of CR845 "resulted in plasma levels at or above the plasma levels of drug expected to be associated with clinical analgesic efficacy" This statement was not backed up by data to make clear why exactly the plasma level of the low dose was expected to lead to clinical analgesia; plasma levels reached after administration of IV CR845 were also not given. Some months earlier, the poster on the preclinical profile did also not include any plasma level related to the effects of CR854 in the various pain models. Thus, the facts (plasma data) were directly translated into strategic relevant information (expectation of clinical analgesia). Company information on CR845 is currently the only driver and base for the opinion of analysts, advising life science investors as well as the base for the stock market trading CARA stocks. The assessments of analysts seem to be positive, to date strong recommendations are given for ‘buy’ by a number of analysts. The absence of peer reviewed articles, for instance reporting all data of the completed phase Ila study in uremic pruritus (data available since 23 July 2015), is a concern. Such data would help to better evaluate the full potential of this new lead in the class of peripheral kappa opiate agonists.
We have demonstrated, via the company communications that most statements are without hedges. Furthermore, in many press releases there can be identified 'spin', positive wording supporting a positive outlook for the compound. Spin and absence of hedges in such communications are most optimally put in context by peer reviewed full papers. Such papers are missing to date. This makes an objective evaluation of the compound's perspective for analysts and scientists difficult. Having said that, CR845 seems to be a promising principle, especially in the field of uremic pruritus was no effective and safe standard treatment exists. Whether a peripheral acting opioid agonist will be active in chronic indications, where we postulate central sensitization as an important pathogenetic factor for pain remains to be seen.
Conclusion
The results of pharmacological, phase I, pilot and pivotal trials of the new peripheral acting kappa opiate agonistCR845 have been communicated in a period of 9 years since start of development in 2008, exclusively by press releases and posters of company representatives. The entire profile of this interesting drug therefore has to be created based on those publications, all written by authors with a conflict of interest. This is not as standalone case, earlier we published on a comparable situation, when we analyzed the data available on the pharmacology of NVA1309, a Novela251 agonist for Neuropathic Pain [23]. Full papers on CR845 in peer reviewed journals are absent to date. In the company communications, many key elements are missing, for instance the numbers of drop-outs, and whether analyses were positive in the ITT population, or in completers. One study, initially, in 2013, was presented as a study with statistically significant findings. Some years later, in 2015, it appeared that this was only the case for the complete analysis; the ITT analysis did not reach significance.
For the other studies, no specifications of the analysis were given. The commentaries of company executives on the findings were also analyzed, and clearly hedges were extremely rare; in most of the cases the statements were quite robust, without careful wording. We feel it is not an optimal situation to be devoid of peer reviewed papers in 2017, if a new principle is developed in the clinic since 2008, and thus be totally and solely dependent on company based information. However, it need to be said, that the company's track record of completing clinical trials, and the context of data presented in uremic pruritus, support a promising future so far. Based on this case study of CR 845, we strongly recommend for companies developing new treatment principles, to facilitate publications of data in peer reviewed journals as soon as possible. Early press communications need to be released, according to the public reporting obligation of the company, but should not stay too long the sole vehicles for the communication of new scientific facts. Peer reviewed articles supply an important new context into the field of knowledge around a new drug, a context also tremendously important for financial analysts and investors. Mostly this context downplays a number of earlier statements, and positions facts in a new, more realistic context.
References
- Science Business (2015) BIG SCIENCE: What's It Worth? Science Business Publishing Ltd, Belgium.
- Coughlin SS, Barker A, Dawson A (2012) Ethics and scientific integrity in public health, epidemiological and clinical research. Public Health Rev 34(1): 71-83.
- Jan De Winter (2014) Defining Scientific Integrity. Philosophy and Public Policy Quarterly 32(3/4): 29-35.
- Fytton Rowland (2002) The peer-review process. Learned publishing 15(4): 247-258.
- Fletcher RH, Black B (2007) Spin in scientific writing: scientific mischief and legal jeopardy. Med Law 26(3): 511-525.
- Horton R (1995) The rhetoric of research. BMJ 310(6985): 985-987.
- Roland MC (2007) Publish and perish. Hedging and fraud in scientific discourse. EMBO Rep 8(5): 424-428.
- Ter Riet G, Chesley P, Gross AG, Siebeling L, Muggensturm P, et al. (2013) All that glitters isn't gold: a survey on acknowledgment of limitations in biomedical studies. PloS one 8(11): e73623.
- Shinoda K, Hruby VJ, Porreca F (2007) Antihyperalgesic effects of loperamide in a model of rat neuropathic pain are mediated by peripheral delta-opioid receptors. Neurosci Lett 411(2): 143-146.
- He SQ, Yang F, Perez FM, Xu Q, Shechter R, et al. (2013) Tolerance develops to the antiallodynic effects of the peripherally acting opioid loperamide hydrochloride in nerve-injured rats. Pain 154(11): 24772486.
- Barber A, Bartoszyk GD, Bender HM, Gottschlich R, Greiner HE, et al. (1994) A pharmacological profile of the novel, peripherally-selective K-opioid receptor agonist, EMD 61753. Br J Pharmacol 113(4): 13171327.
- Stein C (2013) Targeting pain and inflammation by peripherally acting opioids. Front Pharmacol 4: 123.
- Arendt-Nielsen L, Olesen AE, Staahl C, Menzaghi F, Kell S, et al. (2009) Analgesic Efficacy of Peripheral K-Opioid Receptor Agonist CR665 Compared to Oxycodone in a Multi-modal, Multi-tissue Experimental Human Pain Model Selective Effect on Visceral Pain. Anesthesiology 111(3): 616-624.
- Aldrich JV, McLaughlin JP (2009) Peptide kappa opioid receptor ligands: potential for drug development. AAPS J 11(2): 312-322.
- http://investorshub.advfn.com/Cara-Therapeutics-Inc-CARA-27923
- CARA therapeutics. PIPELINE AND BUSINESS STRATEGY.
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, et al. (2010) Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase IIIrandomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 25(4): 1251-1257.
- Joseph W. Stauffer, Paul J Tiseo, Frederique Menzaghi, Robert H Spencer (2015) CR845, A Novel Peripherally-Acting Kappa Opioid Receptor Agonist, Provides Post-Operative Analgesia as Well as Reduces Post-Operative Nausea and Vomiting. American Society of Anesthesiologists, USA.
- Luis R Gardell, Robert H Spencer, Derek T. Chalmers, Frederique Menzaghi (2008) Preclinical Profile of CR845: A Novel, Long-Acting Peripheral Kappa Opioid Receptor Agonist. USA.
- F Menzaghi, R Spencer, N Abrouk, M Lewis, D Chalmers (2015) CR845, a peripheral kappa opioid, provides better pain relief with less nausea and vomiting than placebo in patients after bunionectomy. The Journal of Pain 16(4): S81.
- Cowan A, Kehner GB, Inan S (2015) Targeting Itch with Ligands Selective for k Opioid Receptors. Handb Exp Pharmacol 226: 291-314.
- Jan M Keppel Hesselink (2017) Moving targets in sodium channel blocker development: the case of raxatrigine: from a central NaV1.3 blocker via a peripheral NaV1.7 blocker to a less selective sodium channel blocker. Journal of Medicine and Therapeutics 1(1): 1-3.
- Jan M Keppel Hesselink (2016) A Novel a2S1 Agonist for Neuropathic Pain: NVA1309 of Novassay. Journal of Pharmacology & Clinical Research 1(5): 555575.
- CARA therapeutics (2008) Cara Therapeutics Announces Successful Completion of Phase I Clinical Trial of Novel Analgesic, CR845. USA.
- CARA therapeutics. Cara Therapeutics Successfully Completes Phase I Study With Oral Formulation Of Its Novel Kappa Opioid Receptor Agonist, CR845. USA.
- https://clinicaltrials.gov/ct2/show/results/ NCT00877799?term=CR845&rank=4
- CARA therapeutics (2012) Cara Therapeutics Reports Positive Results From Phase II Trial Of Novel Peripheral Kappa Agonist, CR845, In Acute Post-Operative Pain. USA.
- CARA Therapeutics (2015) Cara Therapeutics Reports Positive Results from Phase 2 Clinical Trial of Novel Peripherally-Acting Kappa Opioid Receptor Agonist, I.V. CR845, for Post-Operative Pain Following Bunionectomy. USA.
- CARA therapeutics (2015) Cara Therapeutics Initiates Phase 3 Program for I.V. CR845 in Acute Postoperative Pain. USA.
- David Kroll (2014) Trial Shows New Opioid Alternative Could Be Less Addictive. Forbes Pharma and Health care, USA.






























