Natural Products as a Source of Anti-Cancer Lead Compounds: Ginger and Breast Cancer
David R. Wallace*
Oklahoma State University Center for Health Sciences, USA
Submission:May 20, 2016; Published:July 05, 2016
*Corresponding author: David Wallace, Oklahoma State University Center for Health Sciences, Department of Pharmacology and Toxicology, 1111 West 17th Street E-367 Tulsa, Oklahoma 74107-1898 USA, Tel: 1-918-561-1407; Email:david.wallace@okstate.edu
How to cite this article: David R. W. Natural Products as a Source of Anti-Cancer Lead Compounds: Ginger and Breast Cancer. J of Pharmacol & Clin Res. 2016; 1(3): 555564. DOI: 10.19080/JPCR.2016.01.555564
Abstract
Nearly 50% of existing pharmaceuticals have natural origins and improved techniques to mass produce derivatives of active component(s) from natural compounds will increase this percentage. Ginger, indigenous to Southeast Asia and Indian subcontinent, has different uses as food-related consumables and in folk medicine. Biologically active components of ginger consist of gingerols, zingerones, shogaols, and zerumbone which demonstrate anti-cancer effects in a variety of cancer cell lines. Over the last decade, increasing investigation has taken place to determine the anti-cancer potential of gingerol, shogaol, and zerumbone. In general, the cellular signaling pathways that control apoptosis and proliferation have been examined (NF-κB, AP-1, p53, STAT3, Akt, IKK, HER2, ERK, COX, LOX, JNK, multiple cascade enzymes, etc). The ability of these compounds to attenuate the growth and development of breast cancer is of particular interest, yet the data is lacking. In breast cancer, evidence suggests that the pathways by which estrogen exerts negative effects on cell growth, such as increased proliferation, decreased apoptosis, etc, can be blocked by the actions of gingerol, shogaol, or zerumbone. The investigation into this topic has been minimal. Limitations to in vivo use of active components of natural products involve pharmacokinetic shortcomings. Also considered as ‘safe’, the majority of these compounds are plagued by poor bioavailability or low potency. Recently, work is being performed on derivatives of shogaol and zerumbone to increase their potential utility as anti-cancer agents. The interest of our laboratory is to utilize these compounds, maximizing their effectiveness to attenuate breast cancer growth.
Keywords: Ginger; Zerumbone; Functional Food; Gingerol; Shogaol; Inflammation; Apoptosis; Cellular Proliferation; Zingerone
Abbreviations: ADMET: Absorption/Distribution/Metabolism/Elimination/Toxicity; Akt: Protein kinase B (serine/threonine-specific protein); AP-1: Activator Protein 1; COMT: Catechol-O-methyltransferase; COX: Cyclooxygenase; E2, 17β: estradiol; ERα and ERβ: Estrogen Receptor α and β; ERK: Extracellular signal-regulated kinase; GPR30: G protein-coupled receptor 30; GST: Glutathione-S-transferase; HER2: Human Epidermal Growth Factor Receptor 2; IKK: IκBα Kinase; JNK: c Jun N-terminal kinase; LOX: Lipooxygenase; MMP2: Matrix Metalloproteinase-2; MMP9: Matrix Metallopeptidase 9; mTOR: mechanistic target of rapamycin (atypical serine/threonine kinase); NF-κB: Nuclear Factor-κB; NQO1, NAD(P)H: Quinone Oxidoreductase I; PI3K: Phosphatidyl Inositol-4,5-bisphosphate 3-kinase; PPARγ: Peroxisome Proliferator-Activated Receptor γ; STAT3: Signal Transducer and Activator of Transcription; TRAIL: Tumor necrosis factor-Related Apoptosis-Inducing Ligand.
Introduction
Ginger’ can mean many things to many people. Belonging to the family ‘Zingiberaceae’ and genus ‘Zingiber’ the most well-known of the different ginger species is Zingiber officinale which is the root commonly used as a spice, but is also known for its therapeutic actions [1]. Zingiber officinale is usually referred to simply as ‘ginger’, yet other species of ginger are being studied. In other ginger species, the difference is in the ratio of active compounds, for example, Zingiber zerumbet (or “bitter ginger”) contains zerumbone as a major constituent [2]. Zingiber officinale contains various gingerols and shogaols as major active components [3]. Indigenous to areas of Southeast Asia and the Indian subcontinent, ginger from particular regions tends to have different uses in food-related consumables and folk medicine.
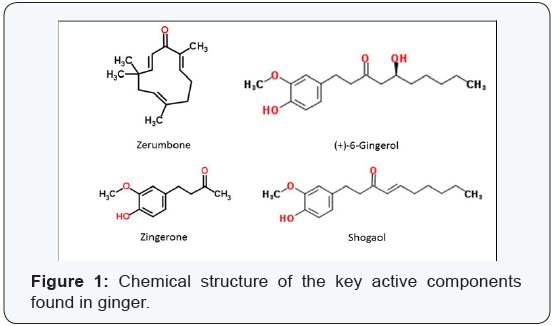
Other spices that are occasionally considered with ginger are cardamom and turmeric, but these are not ‘true’ gingers in that they belong to a different family. For the intent of this mini-review, only the components found in ‘true’ ginger, or Zingiber officinale will be considered. The major constituents of ginger are the gingerols, zingerones, shogaols, and erumbone (Figure 1). These volatile oils and terpenes are what yield the characteristic and sometimes pungent aroma of ginger, whereas the nonvolatile oils give ginger its characteristic flavor. There has been increasing evidence that the active components found in ginger exert positive effects in various cancer cell lines and may provide new lines of drug development for the treatment of different cancers [1]. Even as the evidence comes in, the pathways for these effects are not clearly understood. Additional work needs to be performed to better understand the effects of these compounds on cancer cells. We know that in folk medicine literature, the effects of the active components have been described with little to no associated toxicity. The lack of toxicity suggests that these compounds may be developed as effective anti-cancer agents with significantly reduced adverse effects to the patient.
Within the literature over thousands of years, descriptions of ‘natural products’, ‘functional foods’, ‘nutraceuticals’, etc. have been found in folk medicine documents. The majority of natural products that are discussed are from plant-derived origins. Currently, there is growing subset of the natural product field that involves the development of pharmaceuticals that have a marine origin [4]. Therefore, all of our earliest drugs were of natural origin. Nearly half of our existing drugs have some form of ‘natural’ origin [5]. The use of naturally-derived products as medically active compounds has declined over the last few decades. With the development of new chemistry techniques that could mass produce variations of a molecule’s active backbone (combinatorial chemistry) has limited the perceived need for natural products [6]. The potential for combining the use an active compound, and combinatorial chemistry to produce multiple variations, has been posited as way to advance studies on natural product usefulness in medicine [7-9].
Natural products as anti-tumor agents
Recent work has rejuvenated interest in the anti-cancer ability of compounds from natural products to attenuate the pathogenesis of cancer. Over the last decade, review articles have summarized some of the work that has been performed on natural productmediated anticancer effects [4,9-11]. Review over a dozen natural products as to their anticancer properties. Of which, ginger was one substance reviewed and may function as an anti-cancer agent via increased apoptosis or autophagy. Song et al. [10] focused their review on the chemical moieties which may have a clinical function as anti-cancer agents. They report that nearly 50% of existing anticancer drugs have their origins in a natural product. There is a growing consensus that the use of modern chemistry techniques, in conjunction with the use of active components from natural products, will facilitate not only the development of more efficacious but safer drugs [9,10].
Mechanisms for anti-tumor action
Over the last decade there has been an increased focus on trying to elucidate the mechanisms by which natural products would work to attenuate tumor growth [12-15]. Many of the cellular signaling pathways that control apoptosis and proliferation have been examined (NF-κB, AP-1, p53, STAT3, Akt, IKK, HER2, ERK, COX, LOX, JNK, multiple caspase enzymes, etc.). In many cases, active components from food sources were shown to interfere with not one, but multiple proteins in these pathways [12]. While Aggarwal & Shishodia [12] was a comprehensive review of the current literature, they still caution that more work needs to be done to further clarify the ADMET characteristics of each of the natural product compounds examined. Other groups have begun to investigate pathways with greater specificity to tumor cells such as the up regulation of death receptors on cancer cells [13]. The uniqueness of this pathway is that the death receptors DR4 and DR5 are expressed on cancer cells and up regulating these receptors will increase the efficacy of TRAIL-induced apoptosis, or the active compounds can down regulate pathways necessary for cellular proliferation [13]. Heightened activation of the arachidonic acid pathway, pro-inflammation, is thought to be the precursor to the development of many cancers. Products/compounds which target specific enzymes in the arachidonic acid cascade, such as COX and LOX may be valuable as anti-cancer agents [14]. A review of the underlying causes of many breast cancers involves the same pro-inflammatory pathways [15]. Difficulty in data interpretation is acknowledged by both Aggarwal & Shishodia [12] and Yarla et al. [14]. When one pathway is blocked there is the potential for that pathway to shift, or ‘divert’ to another pathway neutralizing the effect of the active compound. The advantage of many of the active components such as gingerol is that they will block the actions of multiple proteins maximizing their effect. Some question the efficacy and bioavailability of these active compounds such as gingerol. Although generally recognized as safe, there have been few studies addressing the potential toxicity following higher dose, or intravenous administration. It is clear, that much additional work needs to be done to begin to fully understand the mechanistic and biological functions of these active compounds.
Breast cancer and the role of estrogen
Many forms of breast cancer display dependency on the actions of estrogen at its receptors. Estrogen regulation of breast cancer growth may occur by either ligand-receptor interaction on the surface of cells expressing estrogen receptors (GPR30), or by its interaction with the nuclear receptors (ERα and ERβ.). The effects of estrogen, principally E2 (the most potent form of estrogen), have to be analyzed by others and is involved in stimulating cellular proliferation and regulating apoptosis in estrogen receptor-positive cells [16]. Interestingly, although estrogen is implicated in breast cancer, it does have the ability to both stimulate and inhibit tumor growth [16]. It appears that the balance of estrogen action is quite important for determining the outcome, tumor growth or not. Our laboratory is currently studying these effects of estrogen as they relate to receptor stimulate and effects on intracellular signaling mechanisms. The balance of expression/activity of proteins such as p53, caspase, PI3K, Akt, mTOR will be important for determining the ultimate fate of the cell.
Natural products as anti-breast cancer agents
The role of natural products in attenuating the development of breast cancer has begun to be investigated Snelten et al. [17], Bak et al. [18]. Early studies have examined the ability of natural products to reduce the endogenous production of estrogen in estrogen-dependent tumors Snelten et al. [17]. By reducing activity of bioactivating enzymes (aromatase, CYP1B1, CYP1A1), production of estrogen will be reduced. Increasing the activity of enzymes (COMT, NQO1, GST) responsible for estrogen detoxification will result in increased clearance of estrogen and reduced biological effects Snelten et al. [17]. Snelten et al. [17] report that extracts from hops and licorice have displayed the greatest efficacy of the compounds tested to date. This number tested is quite small considering the 100’s of natural products available. These studies have focused on alterations in estrogen synthesis or metabolism; they do not examine the potential intracellular effects of extracts on cellular proliferation. A recent review by Bak et al. [18] has extended the earlier report to include estrogen-mediated mechanisms in estrogen-dependent breast cancers. Of the compounds reviewed, the majority inhibit estrogen-stimulated cellular proliferation. Other systems that are inhibited by the extracts examined are; ERα expression, estrogen response element activity, Akt activity, caspase expression, but increased apoptosis among others Bak et al. [18].
Medical Uses of Ginger and potential as anti-cancer agent
The therapeutic usefulness of ginger has been explored for variety medical needs such as an antimicrobial agent, antioxidant, a neuro-, hepato- and gastro-protective agent, antiemetic agent, and the anti-inflammatory agent [19-21]. An increasing body of evidence suggests that gingerols and shogaols are important compounds for these physiological functions [3]. The role of ginger in the treatment of breast cancer is being investigated in clinical trials but as an anti-nausea agent [22,23]. The route of delivery differs between parental ginger administration in capsule form [22] or as an inhaled agent in aromatherapy [23]. In an animal study, rats that were administered a 0.125% hot water extract of ginger had reduced tumor formation [24]. The ability to suppress inflammation has value regarding reduction in tumor growth. In hepatic cancer, crude ginger extracts reduce the activity of TNFα, thereby inactivating NFκB and reducing inflammatory responses [20]. In cell lines derived from tumors of the head/neck, both 6-gingerol and 6-shogaol reduced cellular proliferation [25]. In gastric cancer cells, 6-gingerol increased apoptosis through both TRAIL induction and inhibition [26]. TRAIL has multiple actions and can increase the activity of caspase 3/7, increasing apoptosis,and can increase NFκB activity via separate mechanisms. There is evidence suggesting the ratio of gingerols to shogaols is important. When comparing the activity of dried to steamed ginger, there was increased anti-tumor potency and a shift towards increasing shogaol ratios in steamed ginger [27]. Extracted gingerol (6-, 8-, and 10-) from ginger was shown to inhibit proliferation of the breast cancer cell line, MDA-MB-231, with a potency order of 10-gingerol > 8-gingerol > 6-gingerol [28]. Interestingly, 10-gingerol was approximately 50-fold more potent (IC50 = 12 versus 670 μM, respectively) than 6-gingerol, which is the form of gingerol normally investigated. Pharmacokinetic analysis in humans demonstrated that 6-gingerol is the best absorbed of the gingerol forms absorption is also better than what was observed for 6-shogaol [29]. Comparing potency with bioavailability will be a consideration for future investigations.
Actions of Gingerols/Shogaols/Zerumbone as antitumor agents
Earlier reports discussed the use of ginger preparations, or of crude extracts to elicit physiological actions. It is now better understood that for Zingiber officinale, it is the gingerols and shogaols that are important [3]. Only recently has there been a research focus on active components of ginger and their ability to act as anti-cancer agents. Gingerols are the most abundant, with 6-gingerol being the most abundant followed by 10-gingerol and 8-gingerol [30]. Multiple cell lines have been utilized while attempting to determine the mechanism of anti-cancer action for gingerols. Epidermoid carcinoma [31], cervical adenocarcinoma [32], prostate [30], breast [33] as well as non-cancer cells [34] have been investigated while attempting to outline the mechanism of gingerol action. Similar to reports for ginger extracts, reductions in cellular proliferation and pro-inflammatory compounds have documented [30,33,34] in cancer cell lines following exposure to gingerol. Gingerol has also been reported to increase apoptosis [32] and reduce oxidative stress by scavenging free radicals [31]. 6-Gingerol exposure reverses the over expression of NFκB, Akt and Bcl2 genes, and increases the expression of the pro-apoptotic genes for TNFα, Bax and cytochrome c [32]. In a breast cancer cell line (MDA-MB-231), exposure to 6-gingerol resulted in an inhibition of metastasis [33]. The activities of both MMP2 and MMP9 were reduced following exposure to 6-gingerol [33]. Both MMP2 and MMP9 are metalloproteins of the Type IV collagenase family and are important for the movement, invasion, and development of breast cancer. It is clear that gingerol has a major role regarding the physiological actions of ginger. The activities are dependent on the preparation of ginger, the relative ratios of the various constituents, and the cell line examined. It is apparent that gingerol exposure elicits multiple actions and can affect the growth of cancer cells via a cascade of intracellular signaling pathways.
The relationship between shogaol and gingerol is established, but recently interest has focused on the function of shogaol alone. In most instances, reports have indicated that 6-shogaol works through pathways similar to 6-gingerol [35-37]. Inhibition of pro-inflammatory pathways via reduced nitric oxide, COX and NFκB are similar to effects observed following 6-gingerol exposure [35,36]. In renal carcinoma cells, 6-shogaol increased TRAIL-induced apoptosis, an effect not observed with 6-gingerol, suggesting cancer-specific effects elicited by the two compounds [37]. Tan et al. [36] suggest that activation of the PPARγ pathway is responsible for the reduction in NFκB activity. Potentially a different pathway compared to the actions of 6-gingerol, the result is the same. Collectively, these finding suggest that the combination of both 6-shogaol and 6-gingerol would provide a multifaceted attack on cancer cells. Interestingly, previous reports indicate that a portion of the shogaol effect is due to generation of free radical and induction of oxidative stress [37,38]. The role of free radical involvement is support by the findings of the oxidative stress-induced release of cytochrome c from the mitochondrial membrane, as well as a reduction in mitochondrial membrane potential [37,38]. Mitochondrial changes are then followed by an up regulation in pro-apoptotic proteins such as caspase 3 and 9 and Bax [38]. Therefore, shogaol exposure can induce apoptosis of cancer cells, through both a similar as well as different pathway compared to 6-gingerol. As previously discussed, drawbacks for the use of biologically active compounds from natural products are the potential for low bioavailability and low potency or efficacy. Using the parent compound as the ‘backbone’ for developing a new derivative may provide a viable avenue regarding future anti-cancer drug development. A 3-phenyl derivative of 3-shogaol was development and displayed actions similar to 6-shogaol with similar potency [35]. Additional studies are needed to determine whether this derivative can separate itself from the use of 6-shogaol by either improved bioavailability or potency.
A general literature search for zerumbone reveal the earliest publications describing zerumbone are from the 1960’s, but many of these reports focused on the cytotoxic actions of zerumbone. Between the mid-1960’s and 2005, the number of publications was sparse. In the last decade, there has been a significant upturn (5-10-fold from previous years) in the number of publications describing the action of zerumbone. Previously, there was little distinction made between zerumbone derived from Zingiber officinale compared to zerumbet. A distinction that is evident in recent publications. Contrary to recent reports regarding the action of 6-shogaol, zerumbone suppresses the production of free radicals, yet the ability to suppress pro-inflammatory agents is similar to what is observed with 6-shogaol [39,40]. A recent review by Prasannan et al. [41] describes the various pathways of zerumbone biological action. In most cases, the mechanism of zerumbone action is similar to what is observed with 6-gingerol and 6-shogaol; with increases in pro-apoptotic protein expression and activity, reductions in proliferative responses, reductions in migratory protein activity, and increases in pro-survival protein expression and activity in normal cells [41]. The anti-cancer action of zerumbone has been examined in multiple cancer cell lines including non-small cell lung [42], ovarian and cervical [43],lymphoblastic leukemia [44] and glioma [45]. Clearly, in each cell line, induction of apoptosis is one of the major mechanisms by which zerumbone attenuates cellular proliferation. Stimulation of p53, a key regulator in the induction of apoptosis, is one site of zerumbone action. In non-small cell lung cancer cells, zerumbone up regulated p53 which induced mitochondrial apoptosis indicated by a reduction in mitochondrial membrane potential, release of cytochrome c and subsequent activation of caspase 3 and 9 [42]. Upstream from p53 activation, zerumbone can suppress the activity of IKKα and Akt, leading to apoptosis [45]. Inhibition of IKKα would suppress NFκB activity downstream, leading to up regulation of caspase 3 and 9. Zerumbone canhalt cellular growth in the G2/M phase preventing mitosis and blocking the secretion of interleukin-6 from cancer cells. Combined with apoptosis, the cellular arrest and interference with interleukin secretion suggest that zerumbone is a potential anti-cancer agent. Similar to recent work with 6-shogaol, new zerumbone derivatives are being developed and examined as anti-cancer agents. An azazerumbone derivative conjugated with chalcones demonstrated increased anti-proliferative potency compared to zerumbone, or azazerumbone alone [46]. These reports further substantiate the viability of zerumbone, or its derivatives, as an anti-cancer agent. To date, minimal investigation into zerumbone effects on breast cancer has occurred. This is an area in need of significant investigation.
Discussion and Conclusion
It is evident that natural products still offer a wealth of potential compounds that will prove to be biological useful for the treatment of cancer. Of particular interest is the potential for new drugs to treat breast cancer. Breast cancer takes the form of many different phenotypes which increase the difficulty in treating these types of cancer. The earliest studies have suggested that ginger in its ‘raw’ (non-extract) form, when used as a tea or in another parental delivery form, may have anti-breast cancer properties. Due to the complicated nature of delineating the action of each ginger active compound, it is hard to draw conclusions from earlier studies. Recent studies using cellular and molecular techniques have begun to elucidate the mechanisms associated with gingerol, shogaol, and zerumbone action. It is evident they inhibit tumor growth in multiple cancer cell lines, yet little work has been done to understand their effects on breast cancer. These compounds, or their derivatives, may all have some level of usefulness in breast cancer therapy. Moreover, once the pathways involved in the pathogenesis breast cancer are paired with the anti-cancer properties of ginger-derived compounds, it may be evident that a combination of compounds would yield a synergistic improvement compared to individual compounds.
Acknowledgement
The author would like to express gratitude to the undergraduate students who helped formulate the idea for this mini-review.
References
- Rahman HS, Rasedee A, Yeap SK, Othman HH, Chartrand MS, et al. (2014) Biomedical properties of a natural dietary plant metabolite, Zerumbone, in cancer therapy and chemoprevention trials. BioMed Res Int 1-20.
- Koga AY, Beltrame FL, Pereira AV (2016) Several aspects of Zingiber zerumbet: a review. Revista Brasileira de Farmacognosia 385–391.
- Semwal RB, Semwal DK, Combrinck S, Viljoen AM (2015) Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 117: 554-568.
- Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH (2005) Marine natural products as anticancer drugs. Mol Cancer Ther 4(2): 333-342.
- Newman DJ, Cragg GM, Snader KM (2003) Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 66(7): 1022-1037.
- Newman DJ (2008) Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem 51(9): 2589-2599.
- Breinbauer R, Vetter IR, Waldmann H (2002) From protein domains to drug candidates-natural products as guiding principles in the design and synthesis of compound libraries. Angew Chem Int Ed Engl 41(16): 2879-2890.
- Breinbauer R, Manger M, Scheck M, Waldmann H (2002) Natural product guided compound library development. Curr Med Chem 9(23): 2129-2145.
- Gordaliza M (2007) Natural products as leads to anticancer drugs. Clin Transl Oncol 9(12): 767-776.
- Song YH, Sun H, Zhang AH, Yan GL, Han Y, et al. (2014) Plant-derived natural products as leads to anti-cancer drugs. J Med Plant Herb Ther Res 6-15.
- Kaur G, Verma N (2015) Nature curing cancer – review on structural modification studies with natural active compounds having antitumor efficiency. Biotech Rep 6: 67-78.
- Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71(10): 1397-1421.
- Prasad S, Kim JH, Gupta SC, Aggarwal BB (2014) Targeting death receptors for TRAIL by agents designed by Mother Nature. Trends Pharmacol Sci 35(10): 520-536.
- Yarla NS, Bishayee A, Sethi G, Reddanna P, Kalle AM, et al. (2016) Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol doi: 10.1016/j.semcancer.
- Suman S, Sharma PK, Rai G, Mishra S, Arora D, et al. (2016) Current perspectives of molecular pathways involved in chronic inflammationmediated breast cancer. Biochem Biophys Res Commun 472(3): 401- 409.
- Lewis-Wambi JS, Jordan VC (2009) Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res 11(3): 206.
- Snelten CS, Dietz B, Bolton JL (2012) Modulation of estrogen chemical carcinogenesis by botanical supplements used for postmenopausal women’s health. Drug Discov Today: Dis Mech 9(1-2): e47-e54.
- Bak MJ, Das Gupta S, Wahler J, Suh N (2016) Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Seminars Cancer Biol (in press) doi: 10.1016/j.semcancer.2016.03.001.
- Rahamani AH, Al Shabrmi FM, Aly SM (2014) Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol Pathophysiol Pharmacol 6(2): 125:136.
- Habib SH, Makpol S, Abdul Hamid NA, Das S, Ngah WZ, et al. (2008) Ginger extract (Zingiber officinale) has anti-cancer and antiinflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo) 63(6): 807-813.
- Shukla Y, Singh M (2007) Cancer preventive properties of ginger: a brief review. Food Chem Toxicol 45(5): 683-690.
- Hickok JT, Roscoe JA, Morrow GR, Ryan JL (2007) A Phase II/III Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Ginger (Zingiber officinale) for Nausea Caused by Chemotherapy for Cancer: A Currently Accruing URCC CCOP Cancer Control Study. Support Cancer Ther 4(4): 247-250.
- Lue PL, Salihah N, Mazlan N (2015) Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med 23(3): 396-404 .
- Nagasawa H, Watanabe K, Inatomi H (2002) Effects of bitter melon (Momordica charantia l.) or ginger rhizome (Zingiber offifinale rosc) on spontaneous mammary tumorigenesis in SHN mice. Am J Chin Med 30(2-3): 195-205.
- Kotowski U, Heiduschka G, Schneider S, Enzenhofer E, Stanisz I, et al. (2015) P25 Effect of bioactive compounds of Zingiber officinale roscoe on head and neck tumor cell lines. Oral Oncol 51: e50.
- Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, et al. (2007) Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Comm 362 (1): 218-223.
- Cheng XL, Liu Q, Peng YB, Qi LW, Li P (2011) Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem 129: 1785-1792.
- Almada da Silva J, Becceneri AB, Sanches Mutti H, Moreno Martin AC, Fernandes da Silva MF, et al. (2012) Purification and differential biological effects of ginger-derived substances on normal and tumor cell lines. J Chromatogr B Analyt Technol Biomed Life Sci 903: 157- 162.
- Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, et al. (2008) Pharmacokinetics of 6-, 8-, 10-gingerols and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers 17(8): 1930-1936.
- Brahmbhatt M, Gundala SR, Asif G, Shamsi SA, Aneja R (2013) Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr Cancer 65(2): 263-272.
- Nigam N, Bhui K, Prasad S, George J, Shukla Y (2009) [6]-Gingerol induces reactive oxygen species regulated mitochondrial cell death pathway in human epidermoid carcinoma A431 cells. Chem Biol Interact 181(1): 77-84.
- Chakraborty D, Bishayee K, Ghosh S, Biswas R, Mandal SK, et al. (2012) [6]-Gingerol induces caspase-3 dependent apoptosis and autophagy in cancer cells: Drug–DNA interaction and expression of certain signal genes in HeLa cells. Eur J Pharmacol 694(1-3): 20-29.
- Lee HS, Seo EY, Kang NE, Kim WK (2008) [6]-Gingerol inhibits metastasis of MDA-MB-231human breast cancer cells. J Nutr Biochem 19(5): 313-319.
- Lee TY, Lee KC, Chen SY, Chang HH (2009) 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun 382(1): 134-139.
- Gan FF1, Ling H, Ang X, Reddy SA, Lee SS, et al. (2013) A novel shogaol analog suppresses cancer cell invasion and inflammation, and displays cytoprotective effects through modulation of NF-κB and Nrf2-Keap1 signaling pathways. Toxicol Appl Pharmacol 272(3): 852-862.
- Tan BS, Kang O, Mai CW, Tiong KH, Khoo AS, et al. (2013) 6-Shogaol inhibits breast and colon cancer cell proliferation through activation of peroxisomal proliferator activated receptor γ (PPARγ). Cancer Lett 336(1): 127-139.
- Han MA, Woo SM, Min KJ, Kim S1 Park JW, et al. (2015) 6-Shogaol enhances renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated cytochrome c release and down-regulation of c-FLIP(L) expression. Chem Biol Interact 228: 69- 78.
- Annamalai G, Kathiresan S, Kannappan N (2016) [6]-Shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependent apoptosis through activation of proapoptotic factors in Hep-2 cells. Biomed Pharmacother 82: 226–236.
- Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, et al. (2002) Zerumbone, a southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the α,β-unsaturated carbonyl group is prerequisite. Carcinogenesis 23(5): 795-802.
- Wang C, Zou S, Cui Z, Guo P, Meng Q, et al. (2015) Zerumbone protects INS-1 rat pancreatic beta cells from high glucose induced apoptosis through generation of reactive oxygen species. Biochem Biophys Res Commun 460(2): 205-209.
- Prasannan R, Kalesh KA, Shanmugam MK, Nachiyappan A, Ramachandran L, et al. (2012) Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem Pharmacol 84(10): 1268-1276.
- Hu Z, Zeng Q, Zhang B, Liu H, Wang W (2014) Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. Biochimie 107: 257-262.
- Abdelwahab SI, Abdul AB, Zain ZN, Hadi AH (2012) Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Inter Immunopharmacol 12 (4): 594-602.
- Abdelwahab SI, Abdul AB, Mohan S, Taha MM, Syam S, et al. (2011) Zerumbone induces apoptosis in T-acute lymphoblastic leukemia cells. Leuk Res 35(2): 268-271.
- Weng HY, Hsu MJ, Wang CC, Chen BC, Hong CY, et al. (2012) Zerumbone suppresses IKKα, Akt and FOX01 activation, resulting in apoptosis of GBM 8401 cells. J Biomed Sci 19: 86.
- Truong VV, Nam TD, Hung TN, Nga NT, Quan PM, et al. (2015) Synthesis and anti-proliferative activity of novel azazerumbone conjugates with chalcones. Bioorg Med Chem Lett 25(22): 5182-5185.







