Hepatoprotective Role of Zinc Sulphate in Carbon Tetrachloride Induced Liver Toxicity
Khalid Aftab*
Isra University, Pakistan
Submission:May 06, 2016; Published:June 23, 2016
*Corresponding author: Khalid Aftab, Professor & HoD PharmacologyAl-Tibri Medical College Old Thana, Gadap Town Malir, Karachi, Pakistan, Tel: 00923002120420; Email:khalidaftabkhan@hotmail.com
How to cite this article: Kashif S, Alina S, Amin F, Khalid A. Hepatoprotective Role of Zinc Sulphate in Carbon Tetrachloride Induced Liver Toxicity. J of Pharmacol & Clin Res. 2016; 1(3): 555562. DOI: 10.19080/JPCR.2016.01.555562
Abstract
Objective: To evaluate the protective role of zinc sulphate in carbon tetrachloride induced hepatic changes in rabbits.
Study Design: Experimental study.
Place of Study: Department of Pharmacology, ISRA University Hyderabad.
Duration of Study: July 2011 to November 2011.
Materials and Methods: A total of 45 rabbits were divided equally into three groups including A, B and C. These groups were further sub divided into (A1, B1 and C1) consisting of 5 animals each who received treatment for one week whereas sub groups (A2, B2 and C2) consisting of 5 animals each received treatment for two weeks and sub groups (A3, B3, and C3) consisting of 5 animals each received treatment for three weeks. The 15 animal included in group A treated as (Control) received normal saline. The 15 animals in group B were treated with carbon tetrachloride (CCl4). The 15 animals in group C were treated with CCl4 along with zinc sulphate.
Result: In present study revealed the hepatotoxic effects on liver morphology showing sinusoidal congestion, periportal inflammation, kupffer cell hyperplasia, steatosis, necrosis and fibrosis produced by carbon tetrachloride. These findings were reduced in rabbits treated with zinc sulphate.
Conclusion: This study concludes that zinc sulphate has hepatoprotective effects in CCl4 induced hepatotoxicity.
Keywords: Carbon Tetrachloride; Zinc Sulphate; Hepatotoxicity; Hepatoprotective
Introduction
CCl4 has probably been Studied more extensively both biochemical and pathologically than any other hepatotoxin. CCl4 catalyzed by CyP450 in the liver cell and yield CCl3 radical. This trichloromethyl radical attack microsomal lipids leading to peroxidation [1]. The experimental intoxication induced by CCl4 is widely used for modeling liver Injury in rats. Hepatotoxicity is connected with severe Impairment of cell protection mechanisms. The location of liver injury is defined mainly by the biotransformation Of CCl4; this is cytochrome P-450 dependant. Free radicals initiate the process of lipid peroxidation, which is generally cause of inhibition of enzyme activity [2]. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system, degenerate the liver [3] and kidneys and may result (after prolonged exposure) in coma and even death [4]. Chronic exposure to carbon tetrachloride can cause liver [5] and kidney damage and could result in cancer [6].
Zinc is an essential mineral that is naturally present in some foods, added to others, and available as a dietary supplement. Zinc is an essential mineral of “exceptional biologic and public health importance” [7]. Lobster and red meats, especially beef, lamb and liver have some of the highest concentrations of zinc in food [8]. Zinc is also found in beans, nuts, almonds, whole grains, pumpkin seeds, sunflower seeds and blackcurrant, Wheat (germ and bran) and various other seeds (sesame, poppy, alfalfa, celery, mustard) [9].
Zinc is involved in numerous aspects of cellular metabolism. It is required for the catalytic activity of approximately 100 enzymes [10]. It is believed to possess antioxidant properties, which may protect against accelerated aging of the skin and muscles of the body; studies differ as to its effectiveness [11]. Zinc could reduce the symptoms of Allergic Rhinitis possibly by decreasing inflammation. Zinc plays a protective role in Age-related macular degeneration (AMD). Zinc deficiency causes alterations in immune response that probably contribute to increased susceptibility to infections, such as those that cause diarrhea, especially in children [12].
The investigation of the mode of action of zinc has also attracted considerable attention recently because of its hepatoprotective effects on hepatic injury involving the inhibition of lipid peroxidation [13], but the mechanisms underlying the antioxidant effects of zinc remain unclear. Several studies on the acute administration of zinc to rats reported a depression of hepatic cytochrome P450 activity associated with a protection against hepatotoxicity This phenomenon was probably, secondary to a decrease in the production of free radical metabolites [14].
Thus the present study is conducted to evaluate the protective role of zinc sulphate in Carbon tetrachloride induced hepatic changes in rabbits.
Materials and Methods
The present experimental study consisted of 45 healthy adult male and female rabbits who were weighing 1.5kg or more. The study was conducted in the Department of Pharmacology, ISRA University Hyderabad. The three groups A, B and C each consisted of 15 animals. Group A was treated as control group whereas Group B was treated with carbon tetrachloride and Group C with carbon tetrachloride along with zinc sulphate.
Each group was further divided into 03sub groups consisting of 05 animals each into (A1, A2 and A3), (B1, B2 and B3) and (C1, C2 and C3).The sub groups (A1, B1 and C1) received treatment for one week and were sacrificed, whereas sub group (A2, B2 and C2) received treatment for two weeks and were sacrificed while. However sub group (A3, B3 and C3) received treatment for three weeks.
Group A: All sub groups (A1, A2 and A3) were treated as controls and were given 0.9% isotonic saline solution at a dose level 4 ml/kg on alternate day. The animals were sacrificed at the end of their respective period of time [15].
Group B: The animals in the sub groups (B1, B2 and B3) were treated with CCl4 dissolved in olive oil (1:1 Ratio) at a dose level of 1.9 ml/kg orally on alternate day. The animals were sacrificed at the end of their respective period of time [15].
Group C: The animals in the sub groups (C1, C2 and C3) were treated with CCl4 dissolved in olive oil at a dose level of 1.9 ml/ kg along with zinc sulphate 1 mg/kg body weight on alternate day. The animals were sacrificed at the end of their respective period of time [16].
At the end of respective period of treatment followed by and sacrifices the animals skin layers and fascia was separated away and liver was identified and removed. The liver tissue specimens were further processed for gross and microscopic examinations. The routine Haematoxyline and Eosin staining was applied and slides were prepared for microscopic examination.
The statistical analysis was carried out by using Chi-square to compare the different histological findings of animals in group A, B and C. The animals were sacrificed at week 1, 2 and 3 to determine the significance at the P value of <0.05considered as significant.
Results
In the present study 45 liver specimens were processed with paraffin embedding and blocks were prepared for staining with Hematoxylin and Eosin. Microscopic examination of liver slides was carried out at 1st, 2nd, 3rd weeks.
- At 1st week: 5 rabbits of sub group A1, B1 and C1 were sacrificed respectively. Normal liver histology was noted in rabbit’s liver treated with isotonic saline. Whereas microscopic examination of the 5 rabbit’s liver treated with CCl4 revealed, sinusoidal congestion and periportal inflammation in two cases and sinusoidal congestion, periportal inflammation and kupffer cell hyperplasia in three animals. Histopathological findings of 5 rabbit’s liver which were given CCl4 along with Zinc sulphate, 3 of them showed no changes and 2 of them showed sinusoidal congestion and periportal inflammation (Table 1).
- At 2nd week: 5 rabbits from sub group A2, B2 and C2 were sacrificed. Normal liver histology was noted in rabbit’s liver treated with isotonic saline. Whereas microscopic examination of the 5 rabbit’s liver treated with CCl4 revealed sinusoidal congestion, periportal inflammation and kupffer cell hyperplasia in three animals while steatosis and piece meal necrosis each was observed in one animal. Histopathological findings of 5 rabbit’s liver given CCl4 along with Zinc sulphate showed normal histology in one animal, 3 of them showed sinusoidal congestion and periportal inflammation and 1 of them showed sinusoidal congestion, periportal inflammation and kupffer cell hyperplasia (Table 2).
- At 3rd week: 5 rabbits from sub group A3, B3 and C3 were sacrificed. Normal liver histology was noted in rabbit’s liver treated with isotonic saline. Whereas microscopic examination of the 5 rabbit’s liver treated with CCl4 revealed steatosis and piece meal necrosis each in one animal, 2 of them showed bridging necrosis and 1 of them showed fibrosis. Histopathological findings of 5 rabbit’s liver treated with CCl4 along with Zinc sulphate showed normal histology in one animal, 3 of them showed sinusoidal congestion and periportal inflammation and 1 of them showed sinusoidal congestion, periportal inflammation and kupffer cell hyperplasia (Table 3). The results revealed that prolong duration of CCl4 exposure for three weeks time has more toxic effects which were also reduced with treatment with zinc sulphate for the longer time.
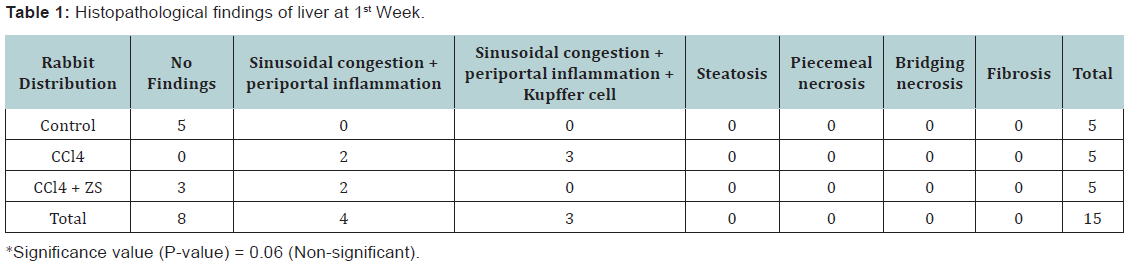

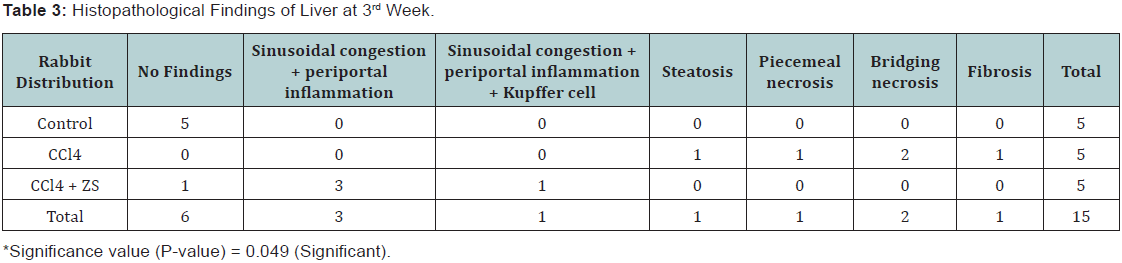
Discussion
The present study revealed the hepatotoxic effects of CCl4 proved by presence of sinusoidal congestion, steatosis, necrosis and fibrosis. These effects are consistent with the findings reported in a study conducted by Weber et al [17]. Cytochrome 2E1 and others convert the CCl4 to CCl3, which in turn binds the various cellular and nuclear molecules thus impairing the lipid metabolism and results in fatty change within the hepatocytes [17]. Khan et al. [18] also reported that CCl4 has damaging effects on the liver. These results correlate with the findings of present study. In present study, Zinc sulphate proved to be hepatoprotective by reducing the toxic effects of CCl4. However, Steven et al. [19] reported that there were no histological changes seen in animal model treated with zinc which is inconsistent with our findings. Goela et al. [20] have reported that zinc improved the histomorphological features of the liver of male rats in chlorpyrifos induced liver injury. This study is consistent with our present study, in which we also observed that zinc reduces steatosis, necrosis and fibrosis in liver. The possible mechanism reducing the toxic effects by zinc treatment was by increasing the antioxidant levels of Glutathione and catalase and also by decreasing the exidant levels of superoxide dismutase [20]. Miranda et al. [21] have reported that zinc appeared to be safe and possibly protective against CCl4 induced hepatotoxicity, the study is consistent with present study.
Conclusion
As in this study, zinc sulphate also played a protective role in CCl4 treated group, which correlate with the study of Rogalska et al. [22] showing that zinc also normalized the CCl4 induced necrosis. Zinc administration resulted in improvement in the structure of hepatocytes in Acetaminophen induced hepatotoxicity in mice [23]. These results are consistent with this study that zinc administration halters the hepatocytes damage.
References
- Wei YH (1998) Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med 217(1): 53-63.
- Poli G, Chiarpotto E, Albano E, Cottalasso D, Nanni G, et al. (1985) Carbon tetrachloride-induced inhibition of hepatocyte lipoprotein secretion: functional impairment of Golgi apparatus in the early phases of such injury. Life Sci 36(6): 533-539.
- Seifert WF, Bosma A, Brouwer A, Hendriks HF, Roholl PJ, et al. (1994) “Vitamin A deficiency potentiates carbon tetrachloride-induced liver fibrosis in rats”. Hepatology 19(1): 193-201.
- Recknagel RO, Glende EA, Dolak JA, Waller RL (1989) “Mechanisms of carbon tetrachloride toxicity”. Pharmacol Ther 43(1): 139-154.
- Masuda Y (2006) Learning toxicology from carbon tetrachlorideinduced hepatotoxicity Article in Japanese. Yakugaku Zasshi 126(10): 885–899.
- Rood AS, McGavran PD, Aanenson JW, Till JE (2001) Stochastic estimates of exposure and cancer risk from carbon tetrachloride released to the air from the rocky flats plant. Risk Anal 21(4): 675-695.
- Hambidge KM, Krebs NF (2007) Zinc deficiency: a special challenge J Nutr 137(4): 1101-1105.
- Berdanier, Carolyn D, Dwyer, Johanna T, Feldman, et al. (2007) Handbook of Nutrition and Food. CRC Press, Boca Raton, Florida, USA.
- Ensminger, Audrey H, Konlande, James E (1993) Foods & Nutrition Encyclopedia (2nd edn). CRC Press, Boca Raton, Florida, USA, pp. 2368– 2369.
- Sandstead HH (1994) Understanding zinc: recent observations and interpretations. J Lab Clin Med 124(3): 322-327.
- Milbury, Paul E, Richer, Alice C (2008) Understanding the Antioxidant Controversy: Scrutinizing the “fountain of Youth” Greenwood Publishing Group p 99.
- Caruso TJ, Prober CG, Gwaltney JM (2007) Treatment of naturally acquired common colds with zinc: a structured review. Clin Infect Dis 45(5): 569-574.
- Camps J, Bargallo T, Gimenez A, Alie S, Caballeria J, et al. (1992) Relationship between hepatic lipid peroxidation and fibrogenesis in carbon tetrachloride-treated rats: effect of zinc administration. Clin Sci (Lond) 83(6): 695-700.
- Arizono K, Sakamoto J, Mikajiri M, Murashima A, Ariyoshi T (1993) Effects of various metals on hepatic biological responses in rat. Trace Elem Med 10: 80.
- Amina E Essawy, Sherifa S Hamed, Ashraf M Abdel Moneim, Ashgan A Abou Gabal, Aglal A Alzergy (2010) Role of black seeds (Nigella sativa) in ameliorating carbon tetrachloride induced haematotoxicity in swiss albino mice. Journal of Medicinal Plants Research 4(19): 1977-1986.
- Batra N, Nehru B, Bansal MP (2004) Reproductive potential of male Portan rats exposed to various levels of lead with regard to zinc status. Br J Nutr 91(3): 387-391.
- Weber LW, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33(2):105-136.
- Rahmat A Khan, Muhammad R Khan, Sumaira Sahreen (2012) CCl4- induced hepatotoxicity: protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complementary and Alternative Medicine 12: 178.
- Steven R Davis, Don A Samuelson, Cousins RJ (2001) Metallothionein Expression Protects against Carbon Tetrachloride-Induced Hepatotoxicity, but Overexpression and Dietary Zinc Supplementation Provide No Further Protection in Metallothionein Transgenic and Knockout Mice. The American Society for Nutritional Sciences.
- Goel A, Dani V, Dhawan DK (2005) Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact 156(2-3): 131-140.
- Miranda CL, Henderson MC, Reed RL, Schmitz JA, Buhler DR (1982) Protective action of zinc against pyrrolizidine alkaloid-induced hepatotoxicity in rats. J Toxicol Environ Health 9(3): 359-366.
- Rogalska J, Pilat Marcinkiewicz B, Brzoska MM (2011) Protective effect of zinc against cadmium hepatotoxicity depends on this bioelement intake and level of cadmium exposure: A study in a rat model. Chem Biol Interact (3): 191-203.
- Chengelis CP, Dodd DC, Means JR, Kotsonis FN (1986) Protection by zinc against acetaminophen induced hepatotoxicity in mice. Fundam Appl Toxicol 6(2): 278-284.







